TRAVERSE Trial Summary
The abstract highlights the findings of the TRAVERSE trial, which aimed to assess the cardiovascular safety of testosterone-replacement therapy in middle-aged and older men with hypogonadism. The trial sought to determine whether testosterone replacement therapy in this population increased the risk of cardiovascular events.
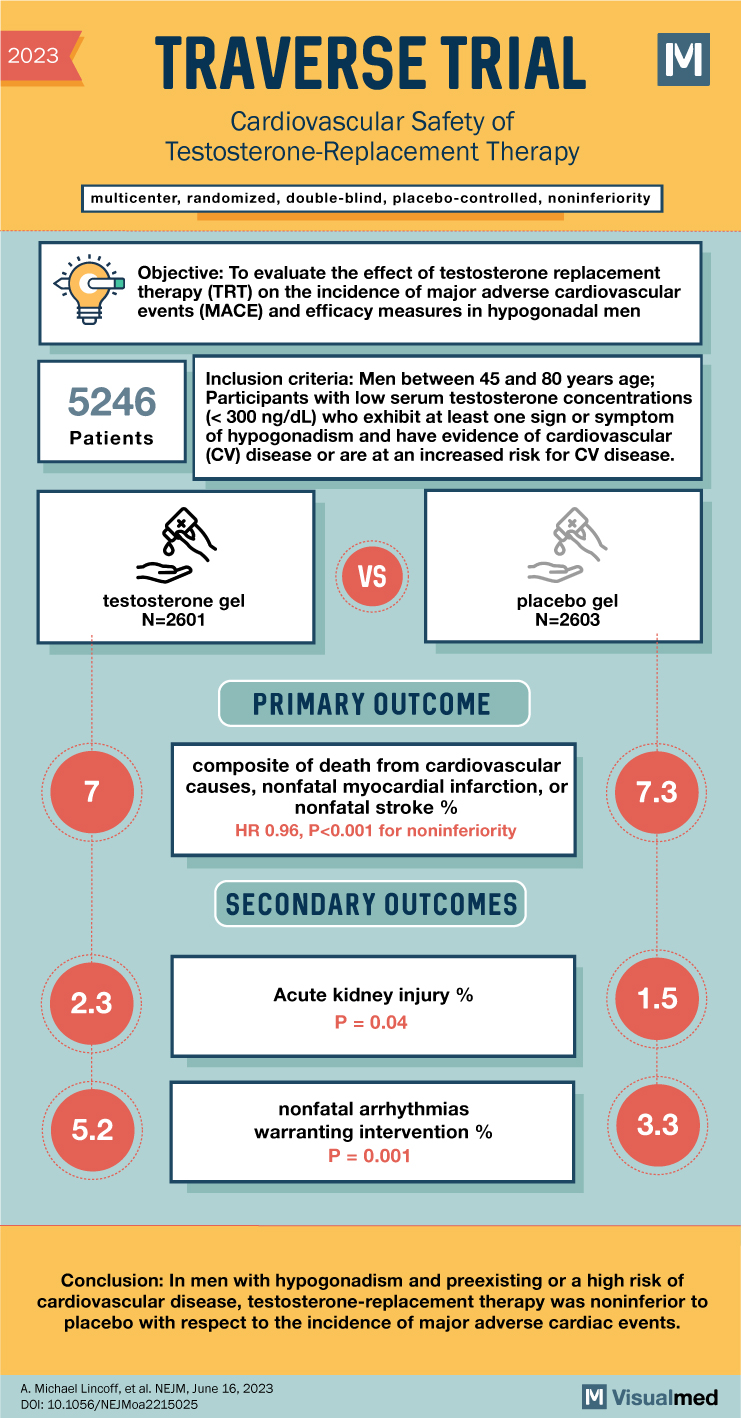
The inclusion criteria for participants required them to be men between the ages of 45 and 80 with low serum testosterone concentrations (<300 ng/dL), exhibiting at least one sign or symptom of hypogonadism, and having evidence of cardiovascular disease or an increased risk for cardiovascular disease.
Certain exclusion criteria were established, including participants with congenital or acquired hypogonadism for whom long-term placebo therapy would not be appropriate, participants with prostate-specific antigen (PSA) levels exceeding certain thresholds, individuals who had received testosterone therapy in the past six months and were contraindicated for further testosterone therapy, and participants with confirmed testosterone levels below 100 ng/dL, a high body mass index (BMI), elevated hemoglobin A1c (HbA1c) levels, high hematocrit (Hct), reduced estimated glomerular filtration rate (eGFR), and a history of deep vein thrombosis, pulmonary embolism, prostate cancer, or heart failure.
The TRAVERSE trial was a multicenter, randomized, double-blind, placebo-controlled, noninferiority trial. A total of 5,246 men aged 45 to 80 years with preexisting cardiovascular disease or a high risk of cardiovascular disease, symptoms of hypogonadism, and low testosterone levels were enrolled. The participants were randomly assigned to receive daily transdermal 1.62% testosterone gel or placebo gel. The primary cardiovascular safety endpoint was the occurrence of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke. Secondary endpoints included death from cardiovascular causes, nonfatal myocardial infarction, nonfatal stroke, or coronary revascularization.
The mean duration of treatment was 21.7 months, with a mean follow-up period of 33.0 months. The results showed that the occurrence of primary cardiovascular endpoint events was similar in both the testosterone group (7.0%) and the placebo group (7.3%). The hazard ratio for the primary endpoint was 0.96, indicating noninferiority of testosterone therapy compared to placebo. Sensitivity analyses also supported these findings. However, the testosterone group did show a higher incidence of atrial fibrillation, acute kidney injury, and pulmonary embolism.
In conclusion, the TRAVERSE trial demonstrated that testosterone-replacement therapy in men with hypogonadism and preexisting or high-risk cardiovascular disease was noninferior to placebo in terms of major adverse cardiac events. The study found no significant difference in the occurrence of primary or secondary cardiovascular endpoints between the testosterone and placebo groups. However, it should be noted that the testosterone group did show an increased incidence of certain adverse events. Further research and monitoring are necessary to fully understand the cardiovascular safety profile of testosterone-replacement therapy in this population.