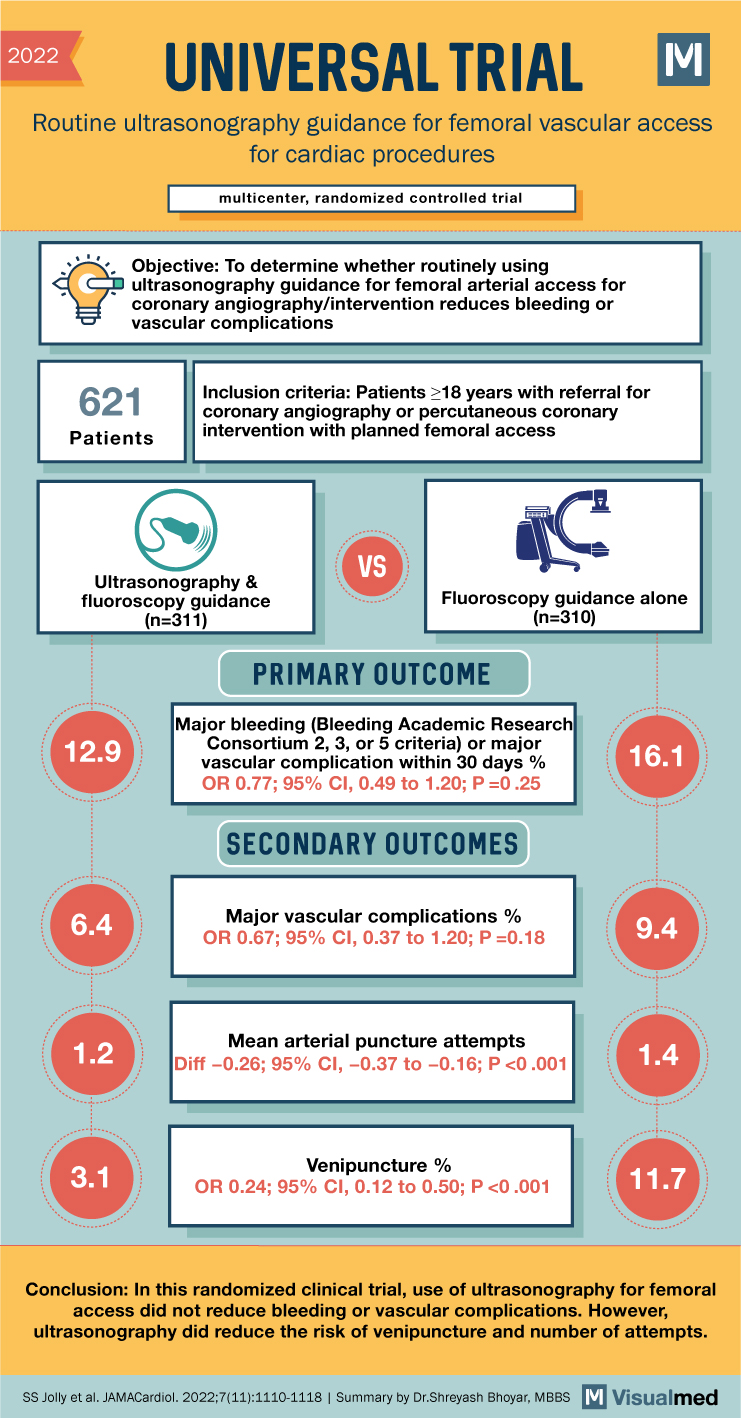
2022 UNIVERSAL TRIAL Routine ultrasonography guidance for femoral vascular access for cardiac procedures multicenter, randomized controlled trial Objective: To determine whether routinely using ultrasonography guidance for femoral arterial access for coronary angiography/intervention reduces bleeding or vascular complications 621 Patients Inclusion criteria: Patients ≥18 years with referral for coronary angiography or percutaneous coronary intervention with planned femoral access TC VS Ultrasonography & Fluoroscopy guidance alone (n=310) fluoroscopy guidance (n=311) 12.9 PRIMARY OUTCOME Major bleeding (Bleeding Academic Research Consortium 2, 3, or 5 criteria) or major vascular complication within 30 days % OR 0.77; 95% CI, 0.49 to 1.20; P=0.25 SECONDARY OUTCOMES 16.1 6.4 Major vascular complications% OR 0.67; 95% CI, 0.37 to 1.20; P=0.18 9.4 1.2 Mean arterial puncture attempts Diff -0.26; 95% CI, -0.37 to -0.16; P <0.001 1.4 3.1 Venipuncture % OR 0.24; 95% CI, 0.12 to 0.50; P <0.001 11.7 Conclusion: In this randomized clinical trial, use of ultrasonography for femoral access did not reduce bleeding or vascular complications. However, ultrasonography did reduce the risk of venipuncture and number of attempts. SS Jolly et al. JAMACardiol. 2022;7(11):1110-1118