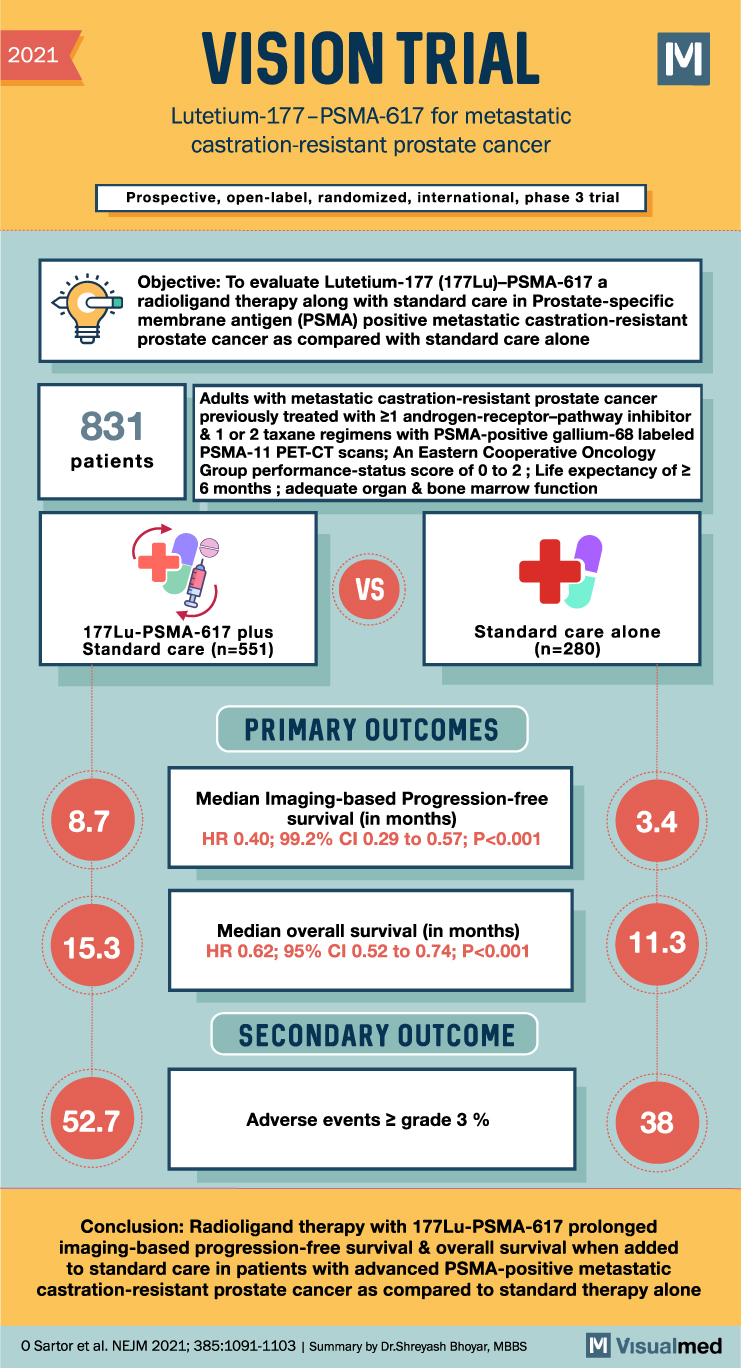
The VISION trial was a phase 3 international, open-label study that aimed to evaluate the efficacy of Lutetium-177 (177Lu)–PSMA-617, a radioligand therapy, in patients with metastatic castration-resistant prostate cancer (mCRPC). This type of cancer remains fatal despite recent advancements, and prostate-specific membrane antigen (PSMA) is highly expressed in mCRPC.
The trial enrolled patients who had previously received treatment with at least one androgen-receptor–pathway inhibitor and one or two taxane regimens and had PSMA-positive gallium-68 (68Ga)–labeled PSMA-11 positron-emission tomographic–computed tomographic scans. The patients were randomly assigned in a 2:1 ratio to receive either 177Lu-PSMA-617 in addition to protocol-permitted standard care or standard care alone. Chemotherapy, immunotherapy, radium-223 (223Ra), and investigational drugs were excluded from the protocol-permitted standard care. The primary endpoints were imaging-based progression-free survival and overall survival, with key secondary endpoints including objective response, disease control, and time to symptomatic skeletal events. Adverse events during treatment were monitored up to 30 days after the last dose and prior to subsequent anticancer treatment.
Between June 2018 and mid-October 2019, a total of 831 out of 1179 screened patients were randomized. The baseline characteristics of the patients were well-balanced between the two groups, and the median follow-up period was 20.9 months. The addition of 177Lu-PSMA-617 to standard care significantly prolonged both imaging-based progression-free survival (median, 8.7 vs. 3.4 months; hazard ratio, 0.40; 99.2% confidence interval [CI], 0.29 to 0.57; P<0.001) and overall survival (median, 15.3 vs. 11.3 months; hazard ratio, 0.62; 95% CI, 0.52 to 0.74; P<0.001) compared to standard care alone. All key secondary endpoints, including objective response, disease control, and time to symptomatic skeletal events, favored the 177Lu-PSMA-617 group. The incidence of adverse events of grade 3 or above was higher in the 177Lu-PSMA-617 group compared to the standard care group (52.7% vs. 38.0%), but the quality of life was not adversely affected.
In conclusion, the VISION trial demonstrated that the addition of 177Lu-PSMA-617 to standard care significantly improved imaging-based progression-free survival and overall survival in patients with advanced PSMA-positive mCRPC. Although there was a higher incidence of grade 3 or above adverse events in the 177Lu-PSMA-617 group, the treatment did not negatively impact the patients’ quality of life. This study provides important evidence for the efficacy of radioligand therapy with 177Lu-PSMA-617 in patients with metastatic castration-resistant prostate cancer and PSMA expression.