VITAL Trial Vitamin D Summary
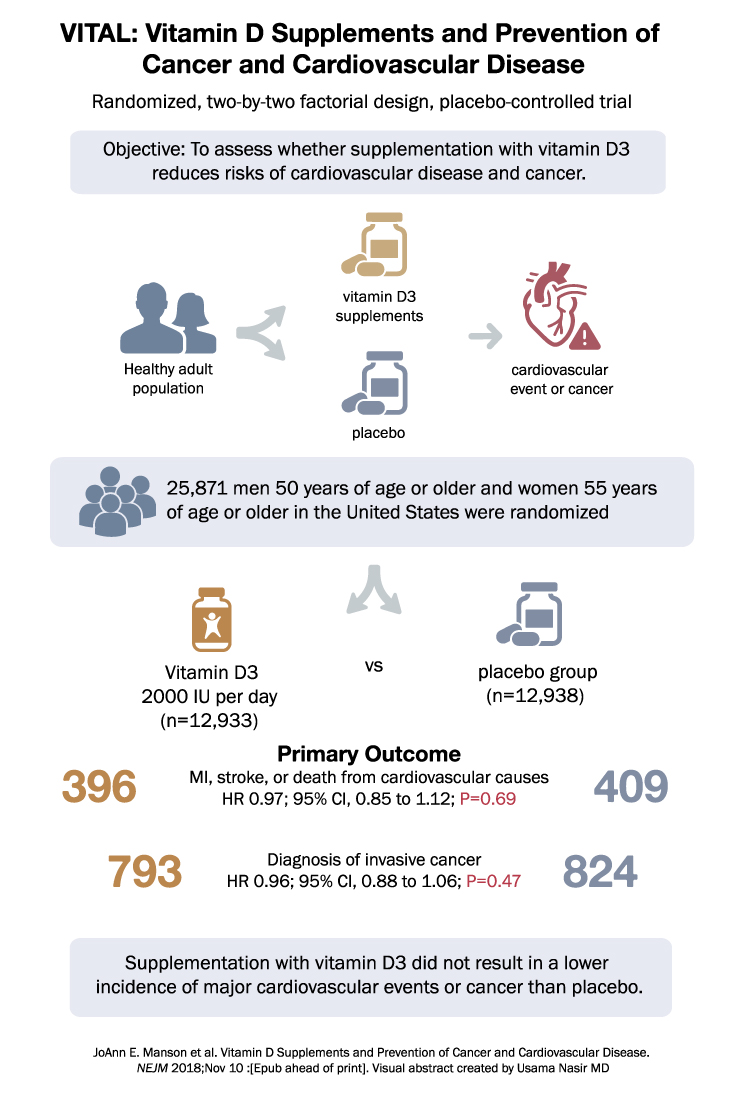
The VITAL trial aimed to investigate whether supplementation with vitamin D reduces the risk of cancer or cardiovascular disease. It was a nationwide, randomized, placebo-controlled trial conducted with a two-by-two factorial design, including vitamin D3 (cholecalciferol) at a dose of 2000 IU per day and marine n−3 (omega-3) fatty acids at a dose of 1 g per day. The trial focused on men aged 50 years or older and women aged 55 years or older in the United States.
The primary endpoints were invasive cancer of any type and major cardiovascular events, which included myocardial infarction, stroke, or death from cardiovascular causes. Secondary endpoints included site-specific cancers, death from cancer, and additional cardiovascular events. This report specifically presents the results of the comparison between vitamin D and placebo.
A total of 25,871 participants, including 5,106 black participants, were randomized and underwent the study. The findings showed that supplementation with vitamin D was not associated with a lower risk of either of the primary endpoints. During a median follow-up of 5.3 years, the incidence of cancer was similar between the vitamin D group and the placebo group, with 793 cases in the vitamin D group and 824 cases in the placebo group (hazard ratio, 0.96; 95% CI, 0.88 to 1.06; P=0.47). Likewise, the occurrence of major cardiovascular events was comparable between the two groups, with 396 events in the vitamin D group and 409 events in the placebo group (hazard ratio, 0.97; 95% CI, 0.85 to 1.12; P=0.69).
The analysis of secondary endpoints, including specific cancer types and additional cardiovascular events, also did not show significant differences between the vitamin D and placebo groups. The hazard ratios for death from cancer, breast cancer, prostate cancer, colorectal cancer, major cardiovascular events plus coronary revascularization, myocardial infarction, stroke, and death from cardiovascular causes were not statistically significant.
The analysis of death from any cause also did not demonstrate a significant difference between the two groups. No excess risks of hypercalcemia or other adverse events associated with vitamin D supplementation were identified.
In conclusion, the VITAL trial found that supplementation with vitamin D at a dose of 2000 IU per day did not lead to a lower incidence of invasive cancer or major cardiovascular events compared to placebo. The results suggest that vitamin D supplementation does not provide substantial benefits in terms of reducing the risk of cancer or cardiovascular disease.
The VITAL trial aimed to investigate whether supplementation with vitamin D reduces the risk of cancer or cardiovascular disease. It was a nationwide, randomized, placebo-controlled trial conducted with a two-by-two factorial design, including vitamin D3 (cholecalciferol) at a dose of 2000 IU per day and marine n−3 (omega-3) fatty acids at a dose of 1 g per day. The trial focused on men aged 50 years or older and women aged 55 years or older in the United States.
The primary endpoints were invasive cancer of any type and major cardiovascular events, which included myocardial infarction, stroke, or death from cardiovascular causes. Secondary endpoints included site-specific cancers, death from cancer, and additional cardiovascular events. This report specifically presents the results of the comparison between vitamin D and placebo.
A total of 25,871 participants, including 5,106 black participants, were randomized and underwent the study. The findings showed that supplementation with vitamin D was not associated with a lower risk of either of the primary endpoints. During a median follow-up of 5.3 years, the incidence of cancer was similar between the vitamin D group and the placebo group, with 793 cases in the vitamin D group and 824 cases in the placebo group (hazard ratio, 0.96; 95% CI, 0.88 to 1.06; P=0.47). Likewise, the occurrence of major cardiovascular events was comparable between the two groups, with 396 events in the vitamin D group and 409 events in the placebo group (hazard ratio, 0.97; 95% CI, 0.85 to 1.12; P=0.69).
The analysis of secondary endpoints, including specific cancer types and additional cardiovascular events, also did not show significant differences between the vitamin D and placebo groups. The hazard ratios for death from cancer, breast cancer, prostate cancer, colorectal cancer, major cardiovascular events plus coronary revascularization, myocardial infarction, stroke, and death from cardiovascular causes were not statistically significant.
The analysis of death from any cause also did not demonstrate a significant difference between the two groups. No excess risks of hypercalcemia or other adverse events associated with vitamin D supplementation were identified.
In conclusion, the VITAL trial found that supplementation with vitamin D at a dose of 2000 IU per day did not lead to a lower incidence of invasive cancer or major cardiovascular events compared to placebo. The results suggest that vitamin D supplementation does not provide substantial benefits in terms of reducing the risk of cancer or cardiovascular disease