ZUMA 7 Trial Summary
Patients with early relapsed or refractory large B-cell lymphoma often face poor prognoses after receiving first-line chemoimmunotherapy. However, a recent international, phase 3 trial has demonstrated promising results with the use of axicabtagene ciloleucel (axi-cel), an autologous anti-CD19 chimeric antigen receptor T-cell therapy.
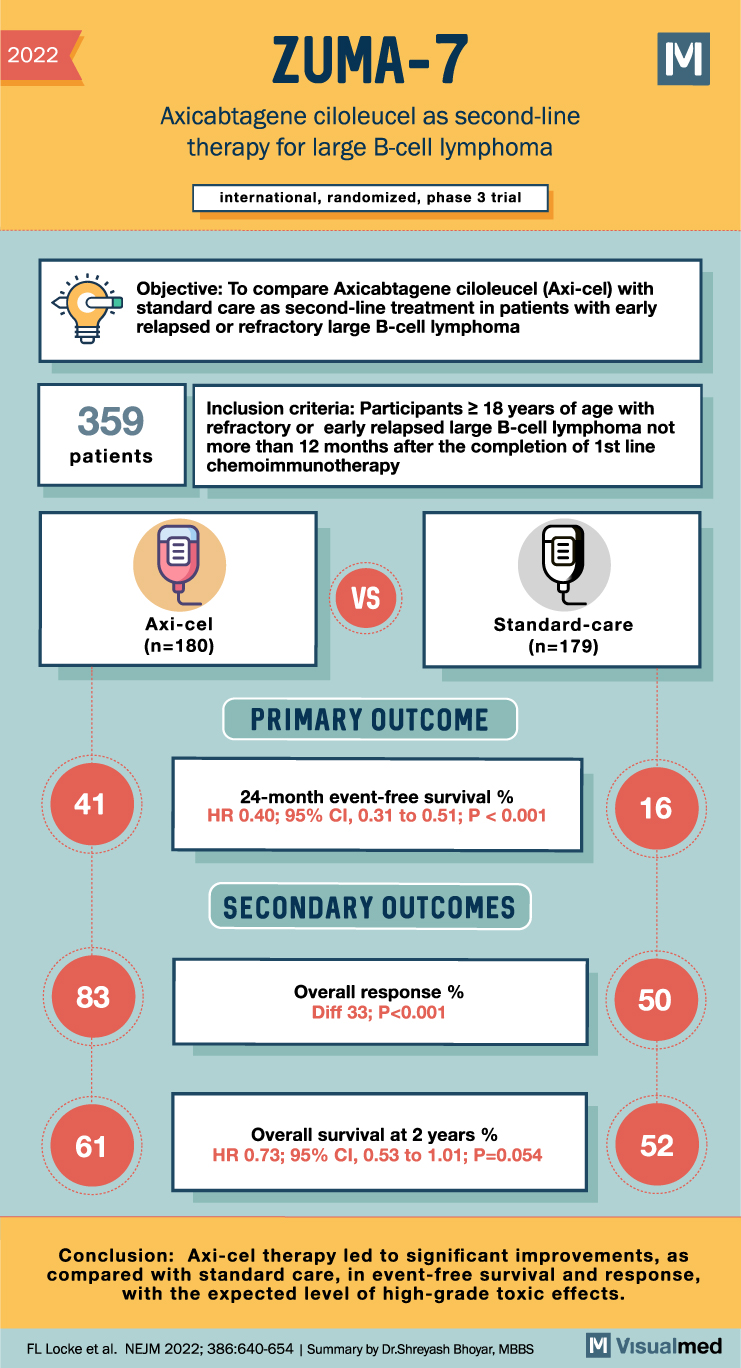
In this trial, patients with large B-cell lymphoma, which had either become refractory to or relapsed within 12 months after the first-line chemoimmunotherapy, were randomized in a 1:1 ratio. One group was assigned to receive axi-cel therapy, while the other received standard care involving two or three cycles of investigator-selected, protocol-defined chemoimmunotherapy, followed by high-dose chemotherapy with autologous stem-cell transplantation in patients responsive to the chemoimmunotherapy. The study aimed to measure event-free survival, response, overall survival, and safety.
With a total of 180 patients assigned to the axi-cel group and 179 to the standard care group, results showed a significant superiority of axi-cel therapy over standard care. At a median follow-up of 24.9 months, the median event-free survival was 8.3 months in the axi-cel group, over four times longer than the 2.0 months in the standard care group. The 24-month event-free survival was also considerably higher in the axi-cel group at 41%, compared to 16% in the standard care group (hazard ratio for event or death, 0.40; 95% confidence interval, 0.31 to 0.51; P<0.001).
Moreover, a response was observed in 83% of patients in the axi-cel group and in only 50% of the standard-care group, with a complete response in 65% and 32% of patients, respectively. In an interim analysis, the estimated overall survival at 2 years was 61% in the axi-cel group and 52% in the standard-care group.
Adverse events of grade 3 or higher were observed in 91% of the patients who received axi-cel and 83% of those who received standard care. Among the axi-cel group, 6% experienced grade 3 or higher cytokine release syndrome and 21% experienced grade 3 or higher neurologic events. However, no deaths related to these adverse events were reported.
In conclusion, axi-cel therapy demonstrated significant improvements in event-free survival and response compared to standard care, despite the expected level of high-grade toxic effects. These results provide new hope for patients with early relapsed or refractory large B-cell lymphoma, and they signal a potentially pivotal development in the treatment of this disease. Further research is required to confirm these findings and refine the use of axi-cel therapy. Source