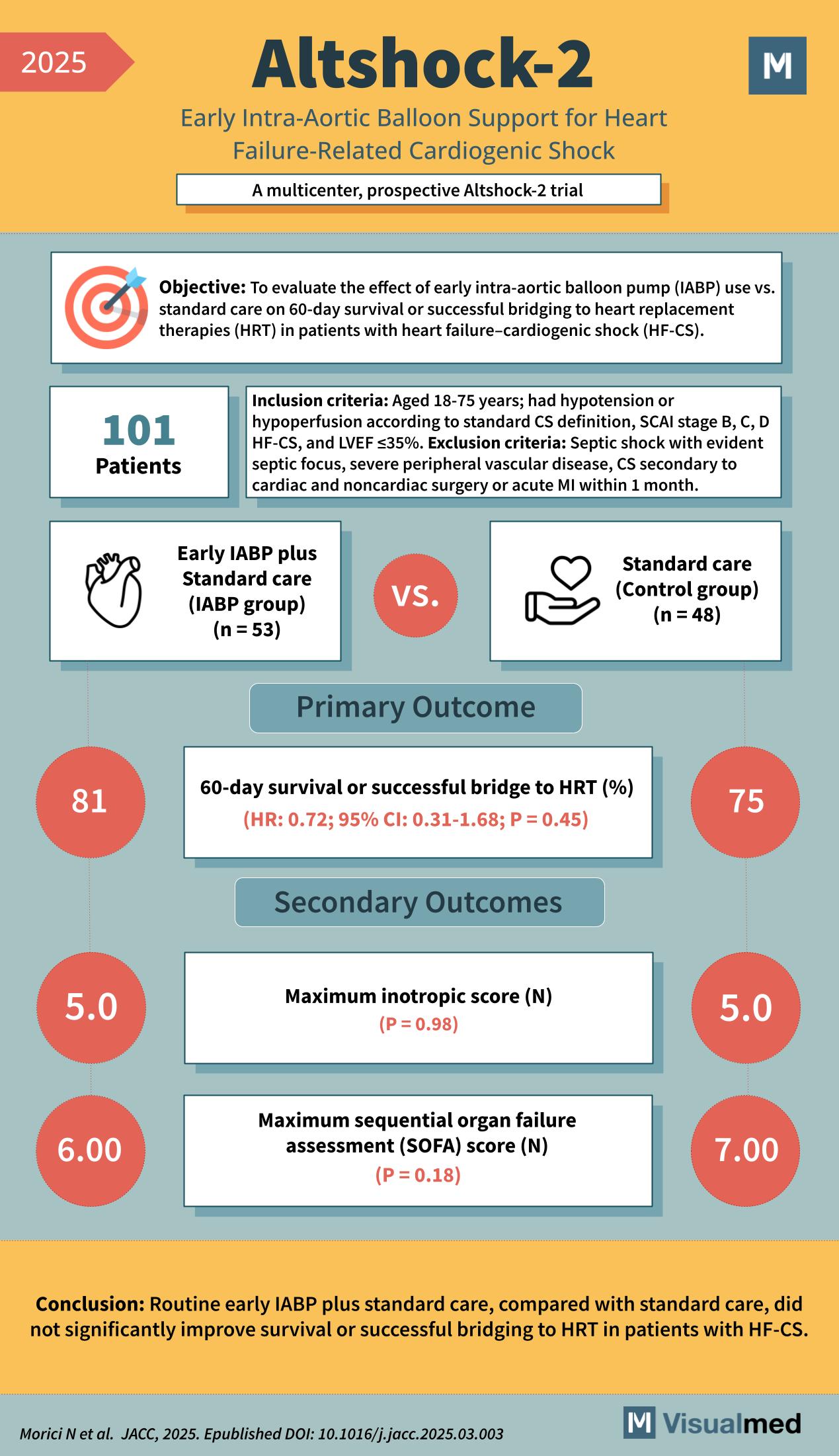
Altshock-2 Trial: Evaluating Early IABP Support in Cardiogenic Shock
The Altshock-2 trial is a multicenter, prospective study designed to evaluate the efficacy of early intra-aortic balloon pump (IABP) support in patients with heart failure-related cardiogenic shock (HF-CS). This trial aimed to determine whether early initiation of IABP, in addition to standard care, improves survival or successful bridging to heart replacement therapies (HRT) in this critically ill population.
A total of 101 patients aged 18–75 years with hypotension or hypoperfusion (SCAI stage B, C, or D) and left ventricular ejection fraction (LVEF) ≤35% were enrolled. Participants were randomized into two groups: 53 received early IABP plus standard care, while 48 received standard care alone. Key exclusion criteria included septic shock, recent cardiac surgery, and severe peripheral vascular disease.
The primary outcome was 60-day survival or successful bridge to HRT. The results showed no significant benefit with early IABP use: 81% of the IABP group met the primary outcome compared to 75% in the control group (Hazard Ratio 0.72; 95% CI: 0.31–1.68; P = 0.45).
Secondary outcomes included the maximum inotropic score (median 5.0 for both groups; P = 0.98) and maximum Sequential Organ Failure Assessment (SOFA) score, which trended slightly lower in the IABP group (6.00 vs. 7.00; P = 0.18), though this was not statistically significant.
Conclusion: The Altshock-2 trial concludes that early routine IABP use in HF-CS does not significantly improve survival or bridging to advanced therapies. These findings suggest that IABP should not be routinely used early in the course of HF-CS and reinforces the need for more effective mechanical support strategies.
Citation: Morici N et al. JACC, 2025. DOI: 10.1016/j.jacc.2025.03.003