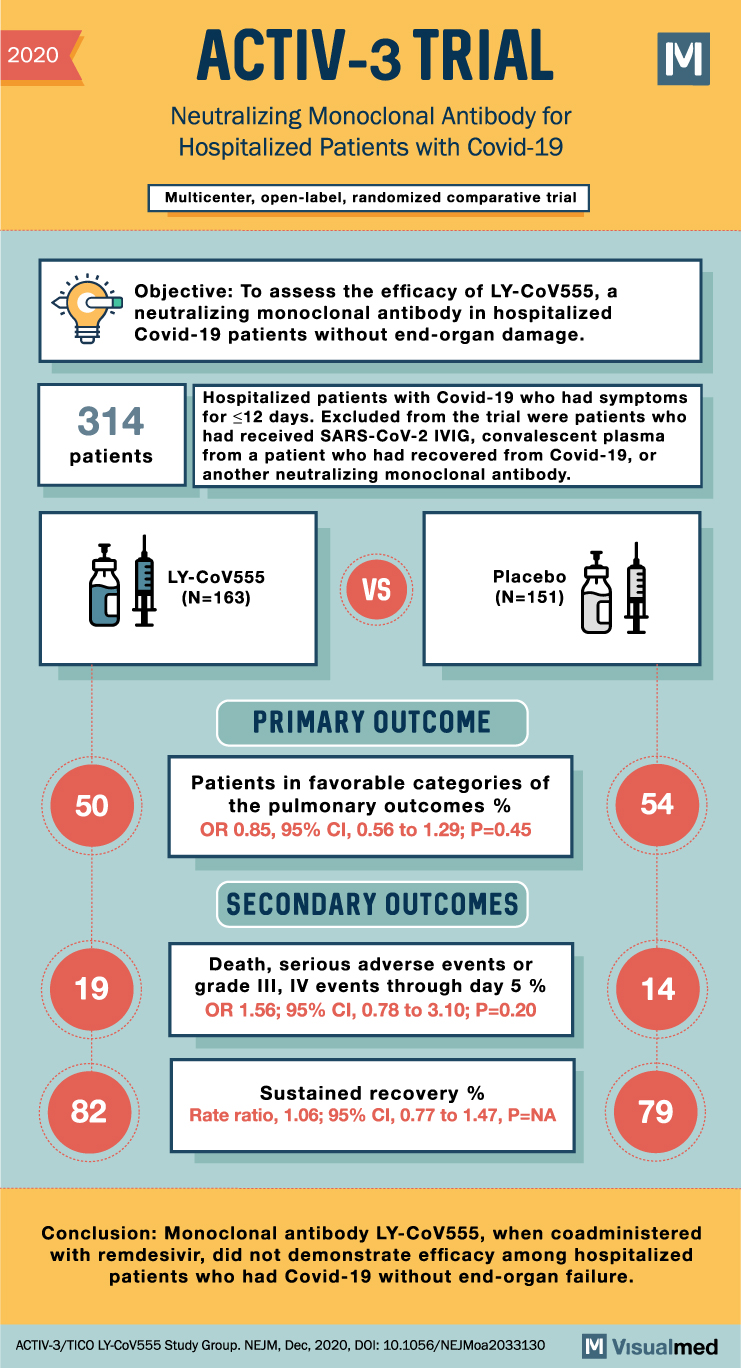
2020 ACTIV-3 TRIAL Neutralizing Monoclonal Antibody for Hospitalized Patients with Covid-19 Multicenter, open-label, randomized comparative trial M Objective: To assess the efficacy of LY-CoV555, a neutralizing monoclonal antibody in hospitalized Covid-19 patients without end-organ damage. Hospitalized patients with Covid-19 who had symptoms 314 for ≤12 days. Excluded from the trial were patients who patients had received SARS-CoV-2 IVIG, convalescent plasma from a patient who had recovered from Covid-19, or another neutralizing monoclonal antibody. 50 LY-CoV555 (N=163) VS Placebo (N=151) PRIMARY OUTCOME Patients in favorable categories of the pulmonary outcomes % OR 0.85, 95% CI, 0.56 to 1.29; P=0.45 SECONDARY OUTCOMES 54 19 Death, serious adverse events or grade III, IV events through day 5 % OR 1.56; 95% CI, 0.78 to 3.10; P=0.20 14 Sustained recovery % 82 Rate ratio, 1.06; 95% CI, 0.77 to 1.47, P=NA 79 Conclusion: Monoclonal antibody LY-CoV555, when coadministered with remdesivir, did not demonstrate efficacy among hospitalized patients who had Covid-19 without end-organ failure. ACTIV-3/TICO LY-COV555 Study Group. NEJM, Dec, 2020, DOI: 10.1056/NEJMoa2033130