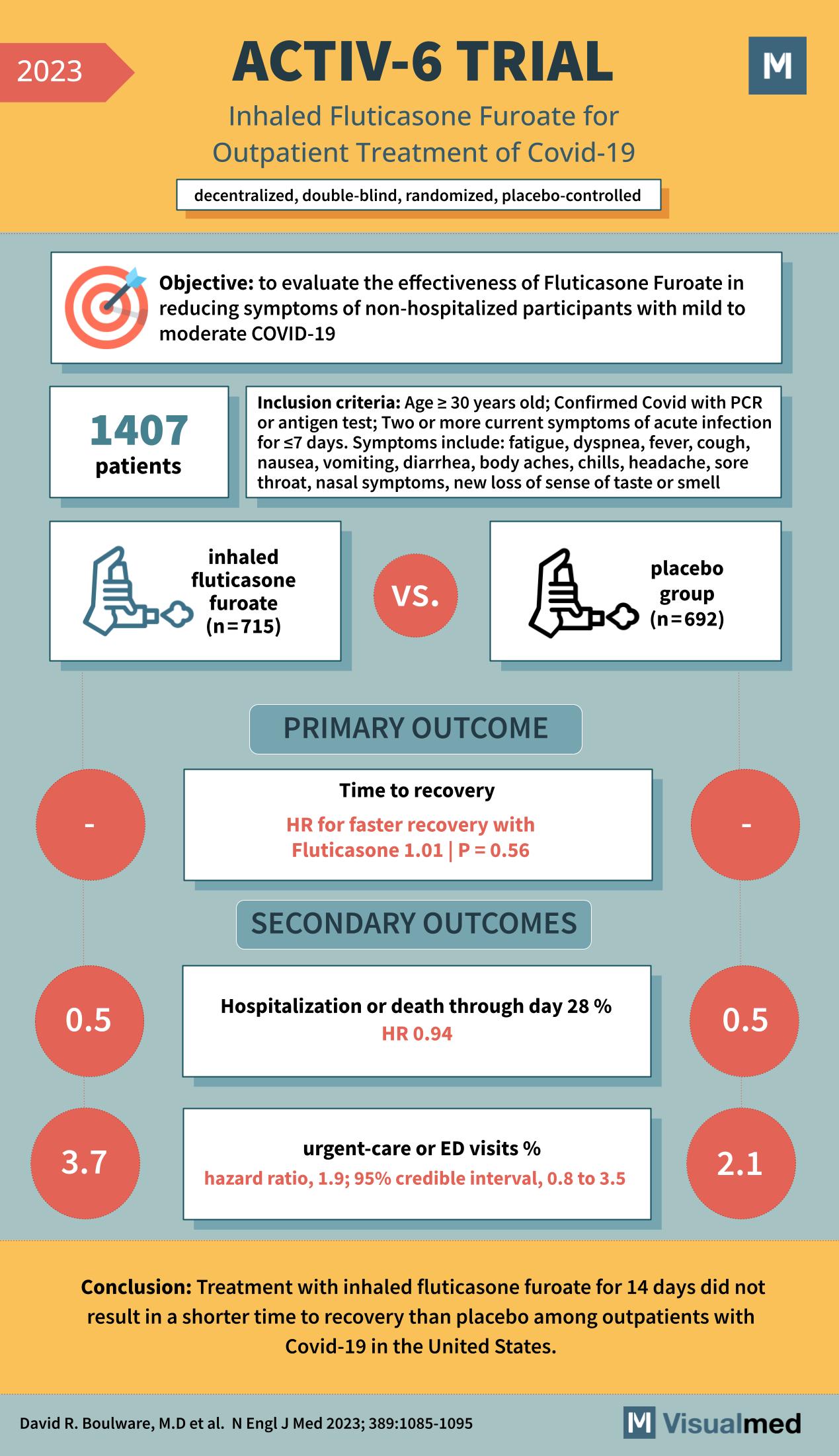
The ACTIV-6 trial, published in the New England Journal of Medicine in 2023, examines the effectiveness of inhaled fluticasone furoate in the treatment of non-hospitalized patients with mild to moderate COVID-19. The trial was a decentralized, double-blind, randomized, placebo-controlled study … Read More
covid 19
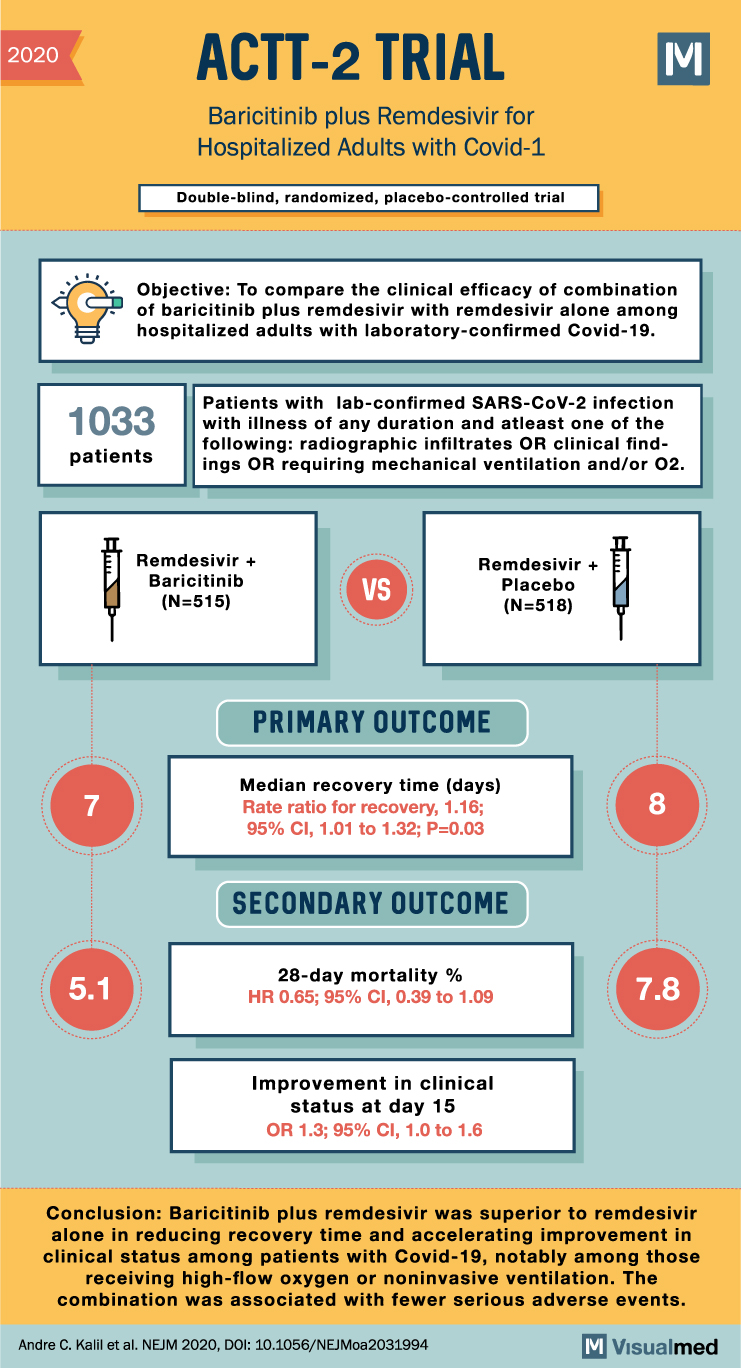
ACTT-2 Trial Summary: Baricitinib + Remdesivir in Covid-19
2020 ACTT-2 TRIAL Baricitinib plus Remdesivir for Hospitalized Adults with Covid-1 Double-blind, randomized, placebo-controlled trial M Objective: To compare the clinical efficacy of combination of baricitinib plus remdesivir with remdesivir alone among hospitalized adults with laboratory-confirmed Covid-19. 1033 patients Patients … Read More
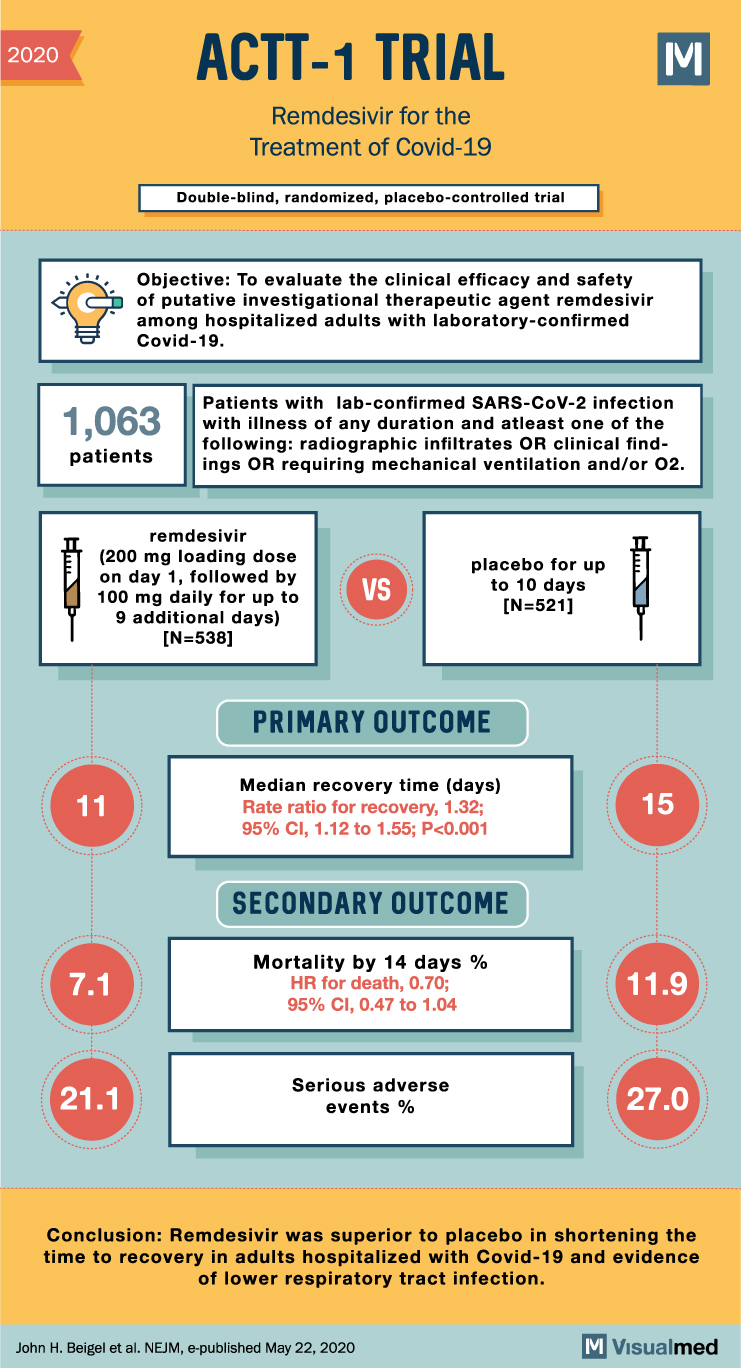
ACTT-1 Trial Summary: Remdesivir in Covid-19
2020 ACTT-1 TRIAL Remdesivir for the Treatment of Covid-19 Double-blind, randomized, placebo-controlled trial Objective: To evaluate the clinical efficacy and safety of putative investigational therapeutic agent remdesivir among hospitalized adults with laboratory-confirmed Covid-19. M Patients with lab-confirmed SARS-CoV-2 infection 1,063 … Read More
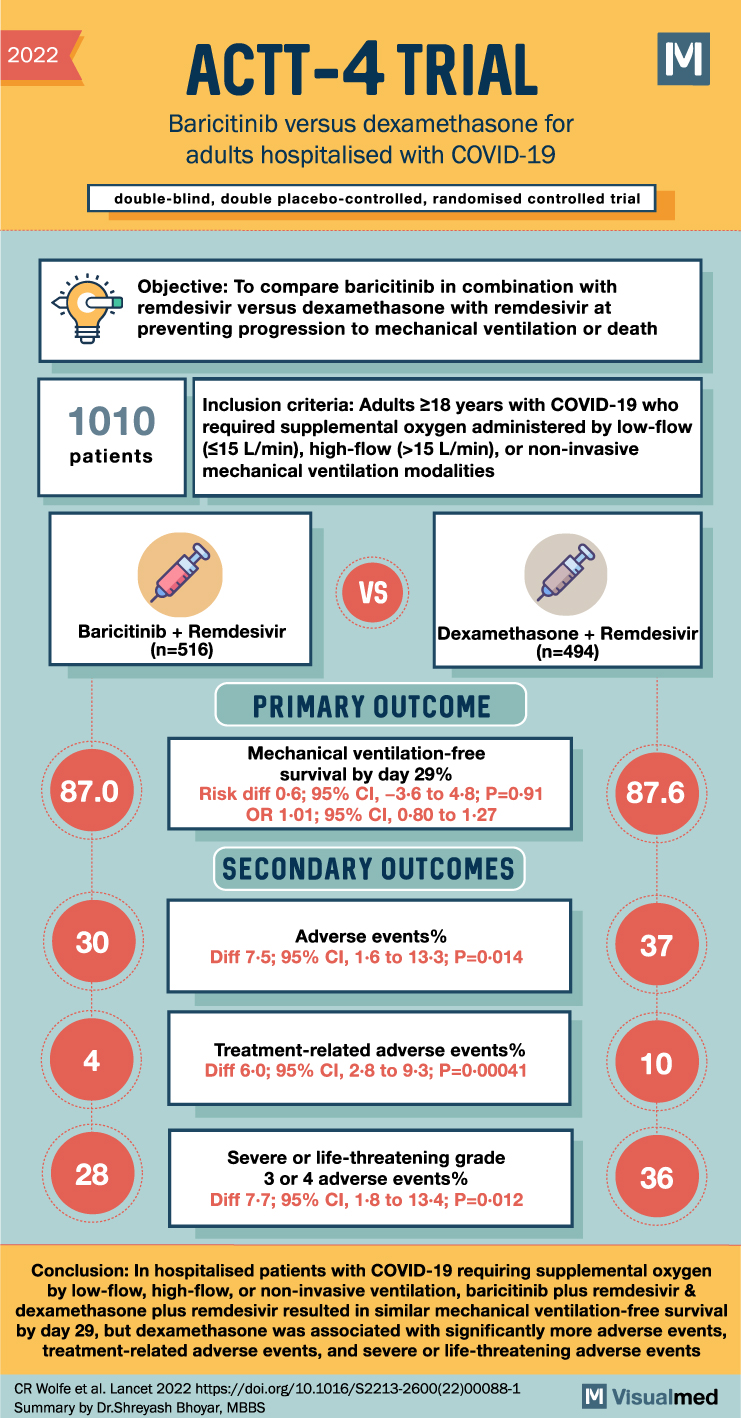
ACTT-4 Trial Summary: Baricitinib in Covid-19
2022 ACTT-4 TRIAL Baricitinib versus dexamethasone for adults hospitalised with COVID-19 double-blind, double placebo-controlled, randomised controlled trial M Objective: To compare baricitinib in combination with remdesivir versus dexamethasone with remdesivir at preventing progression to mechanical ventilation or death 1010 patients … Read More
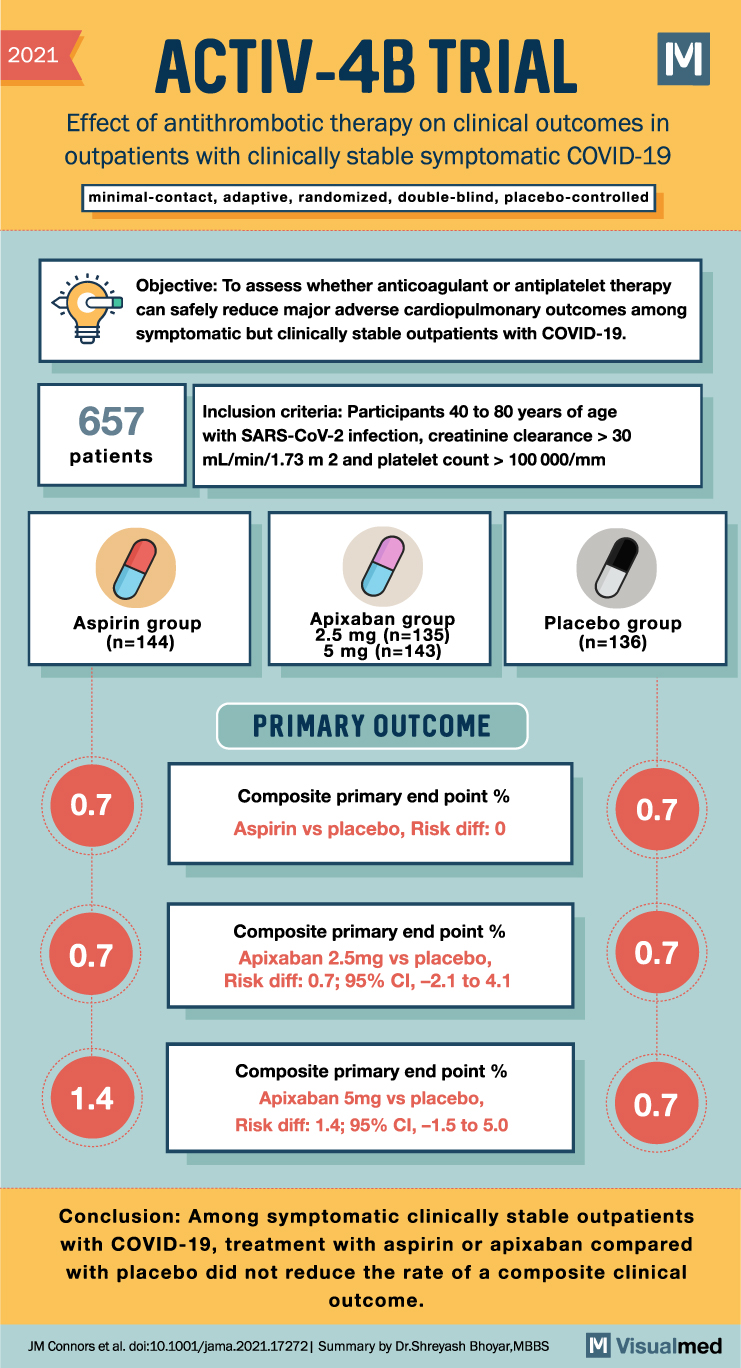
ACTIV-4B Trial Summary: Antithrombotic Therapy in Covid-19
2021 ACTIV-4B TRIAL Effect of antithrombotic therapy on clinical outcomes in outpatients with clinically stable symptomatic COVID-19 minimal-contact, adaptive, randomized, double-blind, placebo-controlled Objective: To assess whether anticoagulant or antiplatelet therapy can safely reduce major adverse cardiopulmonary outcomes among symptomatic but … Read More
ACTIV-3 Trial: Monoclonal Antibody for Covid-19
2020 ACTIV-3 TRIAL Neutralizing Monoclonal Antibody for Hospitalized Patients with Covid-19 Multicenter, open-label, randomized comparative trial M Objective: To assess the efficacy of LY-CoV555, a neutralizing monoclonal antibody in hospitalized Covid-19 patients without end-organ damage. Hospitalized patients with Covid-19 who … Read More
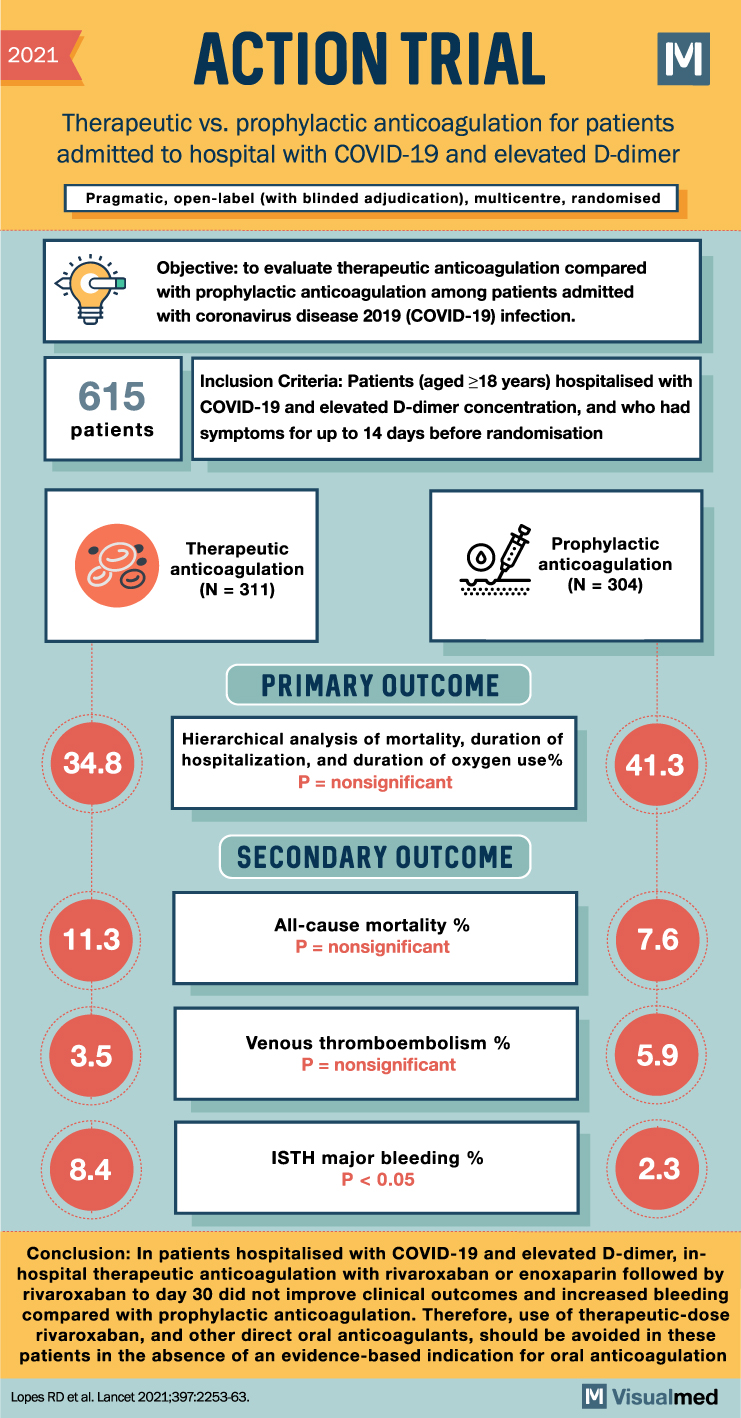
ACTION Trial Summary: Anticoagulation for Hospitalized Covid-19 Patients
2021 ACTION TRIAL: Therapeutic vs. prophylactic anticoagulation for patients admitted to hospital with COVID-19 and elevated D-dimer Pragmatic, open-label (with blinded adjudication), multicentre, randomised Objective: to evaluate therapeutic anticoagulation compared with prophylactic anticoagulation among patients admitted with coronavirus disease 2019 … Read More
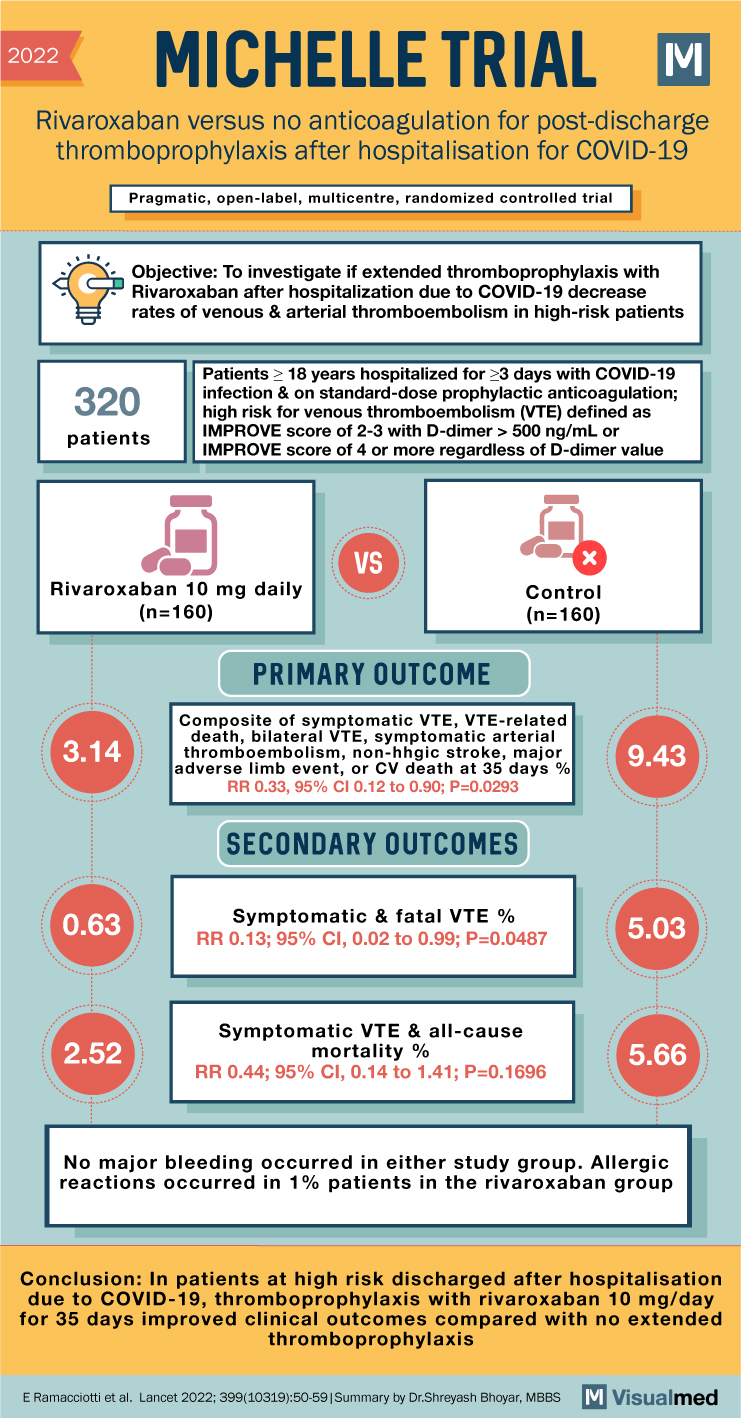
MICHELLE Trial Summary: Rivaroxaban for Thromboprophylaxis in Covid-19
2022 MICHELLE TRIAL Rivaroxaban versus no anticoagulation for post-discharge thromboprophylaxis after hospitalisation for COVID-19 Pragmatic, open-label, multicentre, randomized controlled trial Objective: To investigate if extended thromboprophylaxis with Rivaroxaban after hospitalization due to COVID-19 decrease rates of venous & arterial thromboembolism … Read More