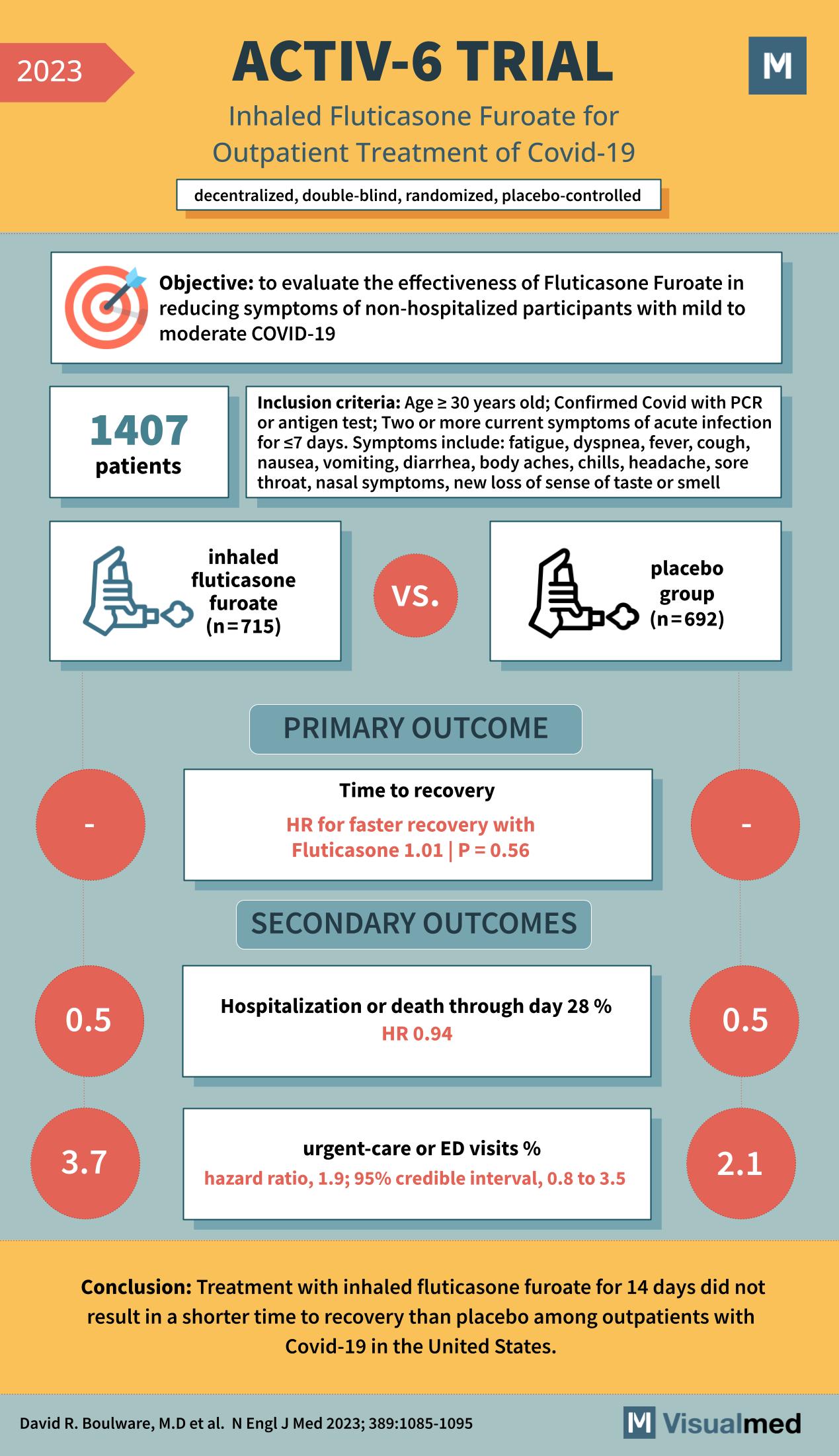
The ACTIV-6 trial, published in the New England Journal of Medicine in 2023, examines the effectiveness of inhaled fluticasone furoate in the treatment of non-hospitalized patients with mild to moderate COVID-19. The trial was a decentralized, double-blind, randomized, placebo-controlled study involving 1,407 patients who were 30 years or older with confirmed COVID-19 and at least two current symptoms of acute infection.
The participants were divided into two groups: those receiving inhaled fluticasone furoate (n=715) and those receiving a placebo (n=692). The primary outcome of the study was the time to recovery, and the results showed no significant difference between the two groups, with a hazard ratio (HR) for faster recovery with fluticasone of 1.01 (p=0.56).
Secondary outcomes included hospitalization or death through day 28 and urgent-care or emergency department (ED) visits. The study found that hospitalization or death rates were similar between the two groups (0.5% in the fluticasone group vs. 0.5% in the placebo group, HR 0.94), indicating no significant benefit of the drug in preventing severe outcomes. However, urgent-care or ED visits were more frequent in the fluticasone group (3.7%) compared to the placebo group (2.1%), with the hazard ratio indicating a higher risk of such visits with fluticasone (HR 1.9; 95% credible interval, 0.8 to 3.5).
The conclusion of the ACTIV-6 trial was that treatment with inhaled fluticasone furoate for 14 days did not result in a shorter time to recovery than the placebo among outpatients with COVID-19 in the United States.
The implications of the ACTIV-6 trial are significant for the management of COVID-19 in outpatient settings. While the treatment did not show the expected benefits in accelerating recovery, the study provides important data on the potential utility and limitations of inhaled corticosteroids in the treatment of COVID-19. It highlights the need for further research to identify effective treatments for the disease and supports the continued evaluation of therapeutic strategies in managing this global health crisis.