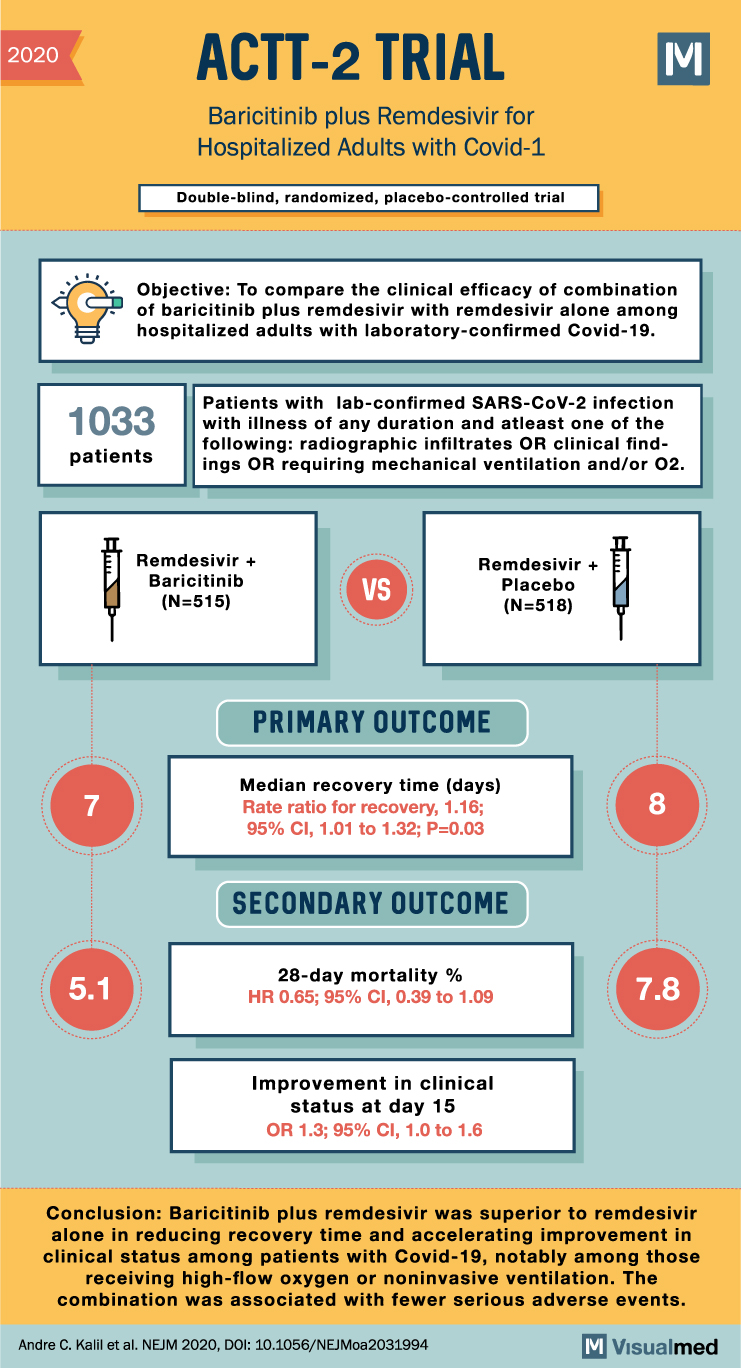
2020 ACTT-2 TRIAL Baricitinib plus Remdesivir for Hospitalized Adults with Covid-1 Double-blind, randomized, placebo-controlled trial M Objective: To compare the clinical efficacy of combination of baricitinib plus remdesivir with remdesivir alone among hospitalized adults with laboratory-confirmed Covid-19. 1033 patients Patients with lab-confirmed SARS-CoV-2 infection with illness of any duration and atleast one of the following: radiographic infiltrates OR clinical find- ings OR requiring mechanical ventilation and/or 02. 7 Remdesivir + Baricitinib (N=515) VS Remdesivir + Placebo (N=518) PRIMARY OUTCOME Median recovery time (days) Rate ratio for recovery, 1.16; 95% CI, 1.01 to 1.32; P=0.03 SECONDARY OUTCOME 8 28-day mortality % 5.1 HR 0.65; 95% CI, 0.39 to 1.09 7.8 Improvement in clinical status at day 15 OR 1.3; 95% CI, 1.0 to 1.6 Conclusion: Baricitinib plus remdesivir was superior to remdesivir alone in reducing recovery time and accelerating improvement in clinical status among patients with Covid-19, notably among those receiving high-flow oxygen or noninvasive ventilation. The combination was associated with fewer serious adverse events. Andre C. Kalil et al. NEJM 2020, DOI: 10.1056/NEJMoa2031994