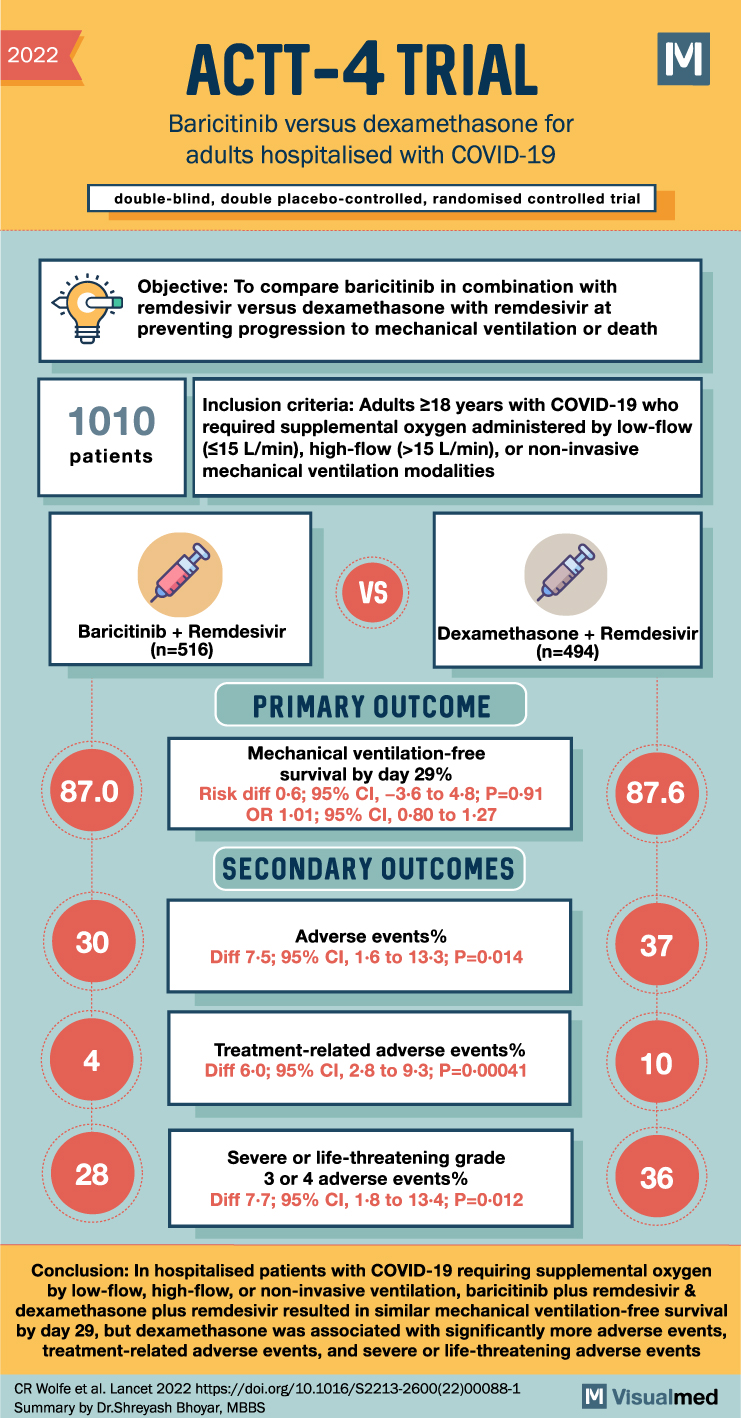
2022 ACTT-4 TRIAL Baricitinib versus dexamethasone for adults hospitalised with COVID-19 double-blind, double placebo-controlled, randomised controlled trial M Objective: To compare baricitinib in combination with remdesivir versus dexamethasone with remdesivir at preventing progression to mechanical ventilation or death 1010 patients Inclusion criteria: Adults ≥18 years with COVID-19 who required supplemental oxygen administered by low-flow ($15 L/min), high-flow (>15 L/min), or non-invasive mechanical ventilation modalities VS Baricitinib + Remdesivir (n=516) Dexamethasone + Remdesivir (n=494) 87.0 PRIMARY OUTCOME Mechanical ventilation-free survival by day 29% Risk diff 0-6; 95% CI, -3-6 to 4.8; P=0.91 OR 1.01; 95% CI, 0.80 to 1.27 SECONDARY OUTCOMES Adverse events% 87.6 30 37 Diff 7-5; 95% CI, 1.6 to 13-3; P=0.014 Treatment-related adverse events% 4 Diff 6-0; 95% CI, 2-8 to 9-3; P=0.00041 10 Severe or life-threatening grade 28 3 or 4 adverse events% Diff 7-7; 95% CI, 1.8 to 13-4; P=0-012 36 Conclusion: In hospitalised patients with COVID-19 requiring supplemental oxygen by low-flow, high-flow, or non-invasive ventilation, baricitinib plus remdesivir & dexamethasone plus remdesivir resulted in similar mechanical ventilation-free survival by day 29, but dexamethasone was associated with significantly more adverse events, treatment-related adverse events, and severe or life-threatening adverse events CR Wolfe et al. Lancet 2022 https://doi.org/10.1016/S2213-2600(22)00088-1