Hypertension remains a major global health issue, particularly for patients whose blood pressure remains uncontrolled despite multiple medications. The Advance-HTN trial, presented at ACC.25 in Chicago in 2025, provides compelling evidence for the use of lorundrostat, a novel aldosterone synthase inhibitor, in treating treatment-resistant hypertension.
What Was the Aim of the Advance-HTN Trial?
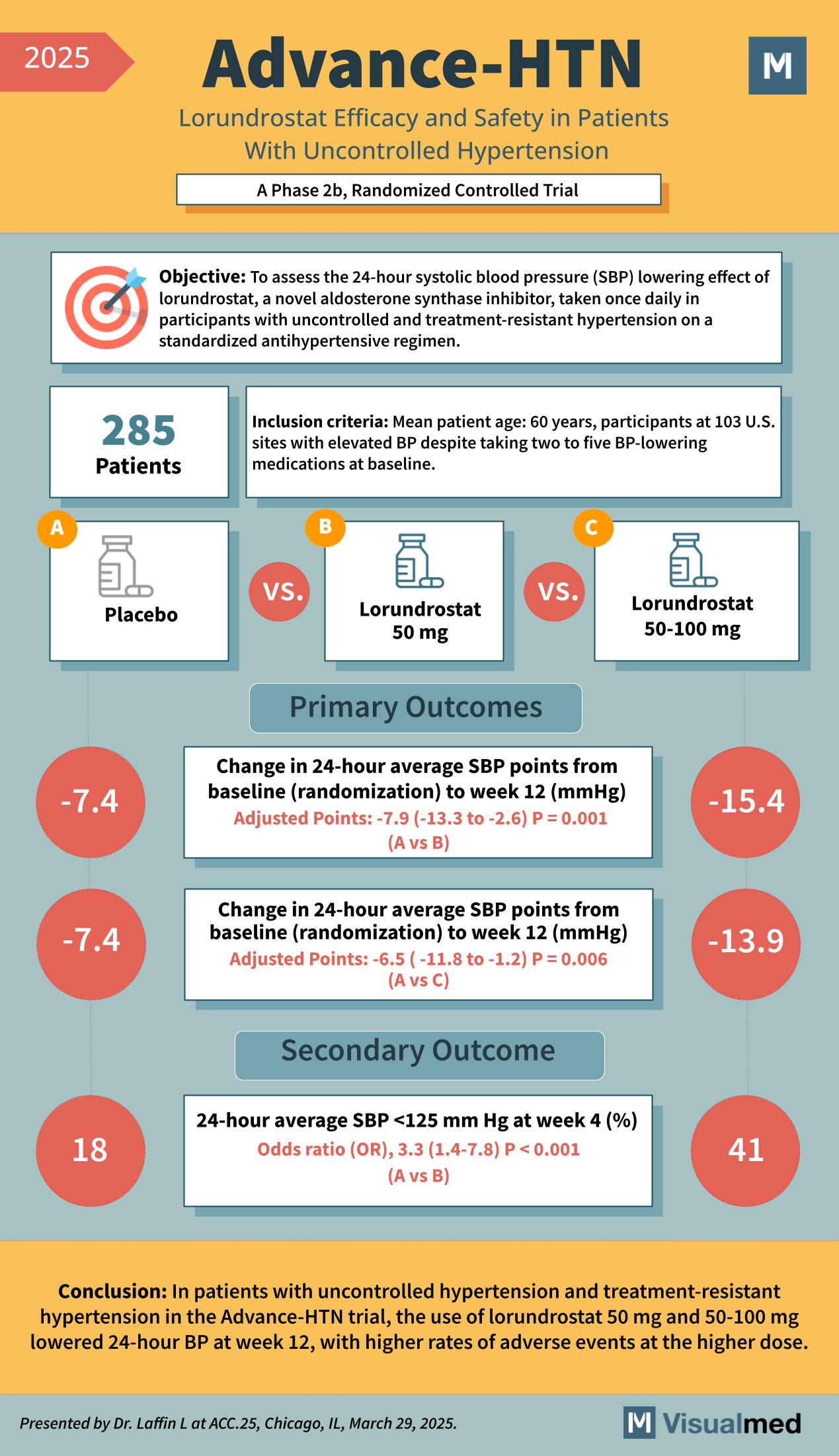
The Advance-HTN trial was a Phase 2b, randomized controlled trial that assessed the effectiveness and safety of lorundrostat in lowering 24-hour systolic blood pressure (SBP) in patients with uncontrolled hypertension. The trial involved 285 patients across 103 sites in the United States. All participants were already on 2 to 5 antihypertensive medications yet still had elevated blood pressure.
Inclusion Criteria
- Mean patient age: 60 years
- Persistent hypertension despite baseline medication use
- Enrolled patients had to be on a stable regimen of blood pressure-lowering drugs
The Study Design
Participants were randomized into three groups:
- Placebo group (A)
- Lorundrostat 50 mg daily (B)
- Lorundrostat 50–100 mg daily (C)
Primary Outcomes
The trial focused on changes in 24-hour average systolic blood pressure (SBP) from baseline to week 12:
- Placebo (A): –7.4 mmHg
- Lorundrostat 50 mg (B): –15.4 mmHg
- Adjusted difference: –7.9 mmHg (95% CI: –13.3 to –2.6), P = 0.001
- Lorundrostat 50–100 mg (C): –13.9 mmHg
- Adjusted difference: –6.5 mmHg (95% CI: –11.8 to –1.2), P = 0.006
These results show that both doses of lorundrostat led to statistically significant and clinically meaningful reductions in blood pressure compared to placebo.
Secondary Outcome
A secondary endpoint examined the percentage of patients achieving 24-hour average SBP <125 mmHg at week 4:
- Placebo: 18%
- Lorundrostat 50 mg: 41%
- Odds Ratio (OR): 3.3 (95% CI: 1.4 to 7.8), P < 0.001
This underscores lorundrostat’s potential to bring a significant proportion of patients under control in just four weeks.
Safety Profile
While effective, the higher dose group (50–100 mg) experienced more adverse events, signaling the need for careful dosing and monitoring in clinical use.
Conclusion
The Advance-HTN trial presents lorundrostat as a promising new option for patients with treatment-resistant hypertension. The drug achieved a substantial drop in 24-hour SBP over 12 weeks and enabled more patients to reach target blood pressure levels. With larger studies and long-term follow-up, lorundrostat could soon become a key player in hypertension management.