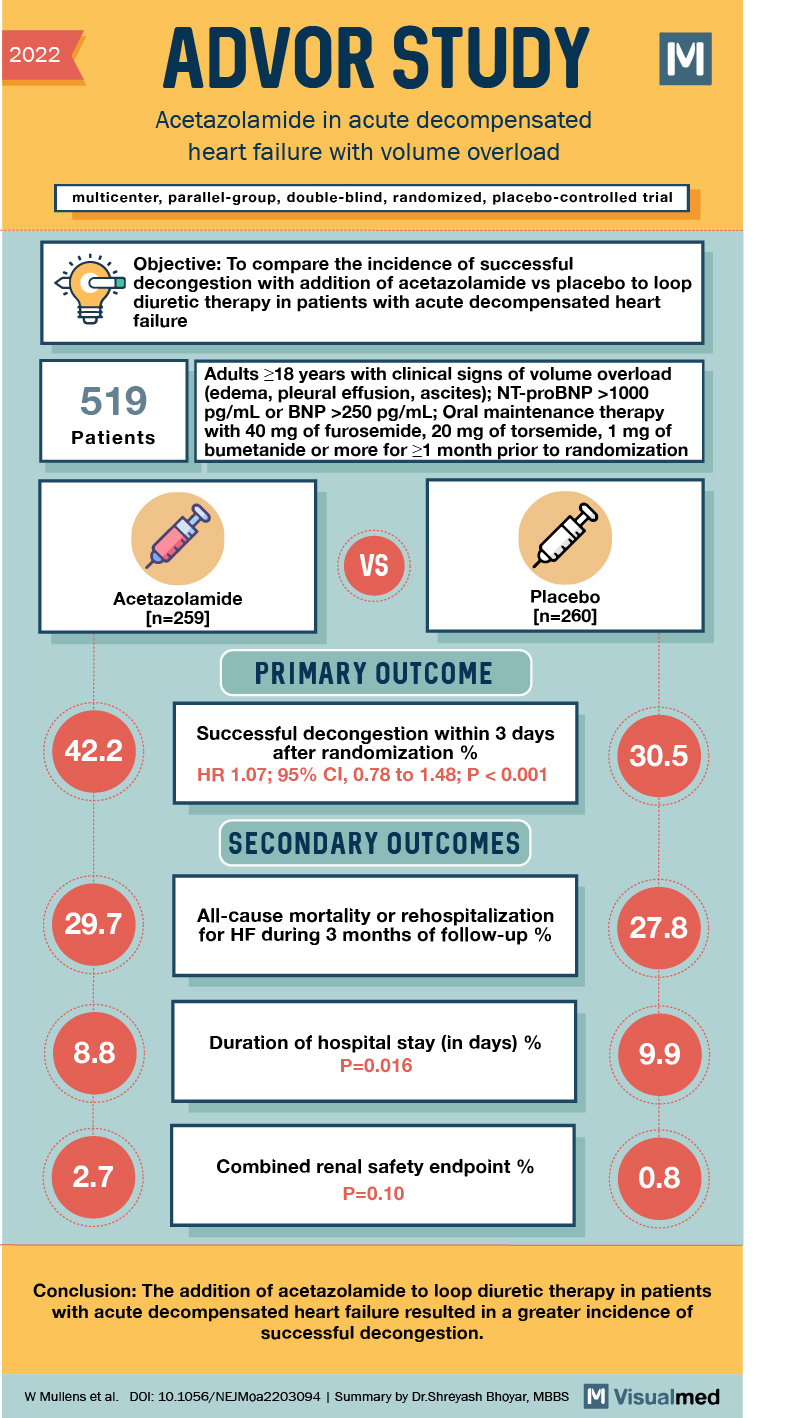
2022 ADVOR STUDY Acetazolamide in acute decompensated heart failure with volume overload multicenter, parallel-group, double-blind, randomized, placebo-controlled trial a d Objective: To compare the incidence of successful decongestion with addition of acetazolamide vs placebo to loop diuretic therapy in patients with acute decompensated heart failure 519 Adults 18 years with clinical signs of volume overload (edema, pleural effusion, ascites); NT-proBNP >1000 pg/mL or BNP >250 pg/mL; Oral maintenance therapy with 40 mg of furosemide, 20 mg of torsemide, 1 mg of bumetanide or more for >1 month prior to randomization Patients VS SEO Acetazolamide
[n=259]
Placebo [n=260] PRIMARY OUTCOME 42.2 Successful decongestion within 3 days after randomization % HR 1.07; 95% CI, 0.78 to 1.48; P < 0.001 30.5 SECONDARY OUTCOMES 29.7 All-cause mortality or rehospitalization for HF during 3 months of follow-up % 27.8 8.8 Duration of hospital stay (in days) % P=0.016 9.9 2.7 Combined renal safety endpoint % P=0.10 0.8 Conclusion: The addition of acetazolamide to loop diuretic therapy in patients with acute decompensated heart failure resulted in a greater incidence of successful decongestion. W Mullens et al. DOI: 10.1056/NEJMoa2203094