The AGENT IDE Trial: Revolutionizing the Treatment of In-Stent Restenosis
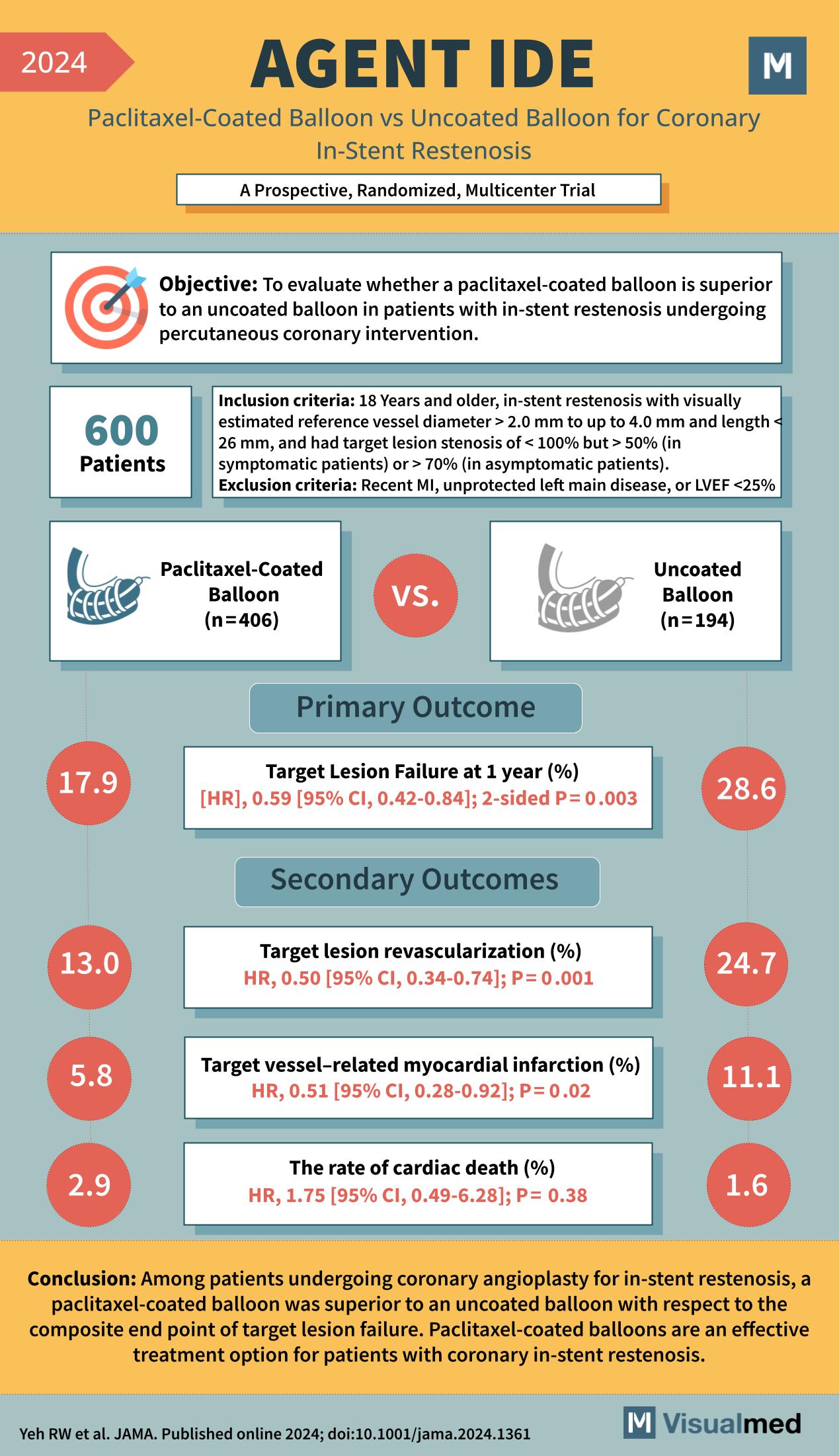
The AGENT IDE Trial, a prominent study published in 2024, has shed light on a pivotal question in cardiology: Is a paclitaxel-coated balloon more effective than an uncoated balloon for treating coronary in-stent restenosis? This prospective, randomized, multicenter trial has garnered attention for its potential to change clinical practices.
Objective of the AGENT IDE Trial
The objective of the AGENT IDE Trial was to evaluate the superiority of a paclitaxel-coated balloon compared to an uncoated balloon in patients with in-stent restenosis undergoing percutaneous coronary intervention.
Trial Enrolment and Criteria
The trial included 600 patients who met the inclusion criteria: aged 18 years and older, diagnosed with in-stent restenosis, and having a reference vessel diameter of 2.0 mm to 4.0 mm and length ≤ 26 mm. The study excluded those who had recently suffered a myocardial infarction (MI), had unprotected left main disease, or had a left ventricular ejection fraction (LVEF) of less than 25%.
Study Design
Patients were divided into two groups, with 406 receiving the paclitaxel-coated balloon and 194 receiving the uncoated balloon.
Results of the AGENT IDE Trial
Primary Outcome
- Target Lesion Failure at 1 year occurred in 17.9% of patients in the paclitaxel-coated balloon group, significantly lower than the 28.6% in the uncoated balloon group, with a hazard ratio (HR) of 0.59 (P = 0.003).
Secondary Outcomes
- Target lesion revascularization was required in 13.0% of the paclitaxel-coated balloon group compared to 24.7% of the uncoated balloon group, with an HR of 0.50 (P = 0.001).
- Target vessel-related myocardial infarction was reported in 5.8% in the paclitaxel-coated balloon group and in 11.1% of the uncoated balloon group, with an HR of 0.51 (P = 0.02).
- The rate of cardiac death was 2.9% in the paclitaxel-coated balloon group and 1.6% in the uncoated balloon group, not significantly different with an HR of 1.75 (P = 0.38).
Conclusion and Implications
The AGENT IDE Trial concluded that paclitaxel-coated balloons are superior to uncoated balloons for the composite endpoint of target lesion failure. The data from the trial strongly suggest that paclitaxel-coated balloons should be considered a viable and effective treatment option for patients with coronary in-stent restenosis.