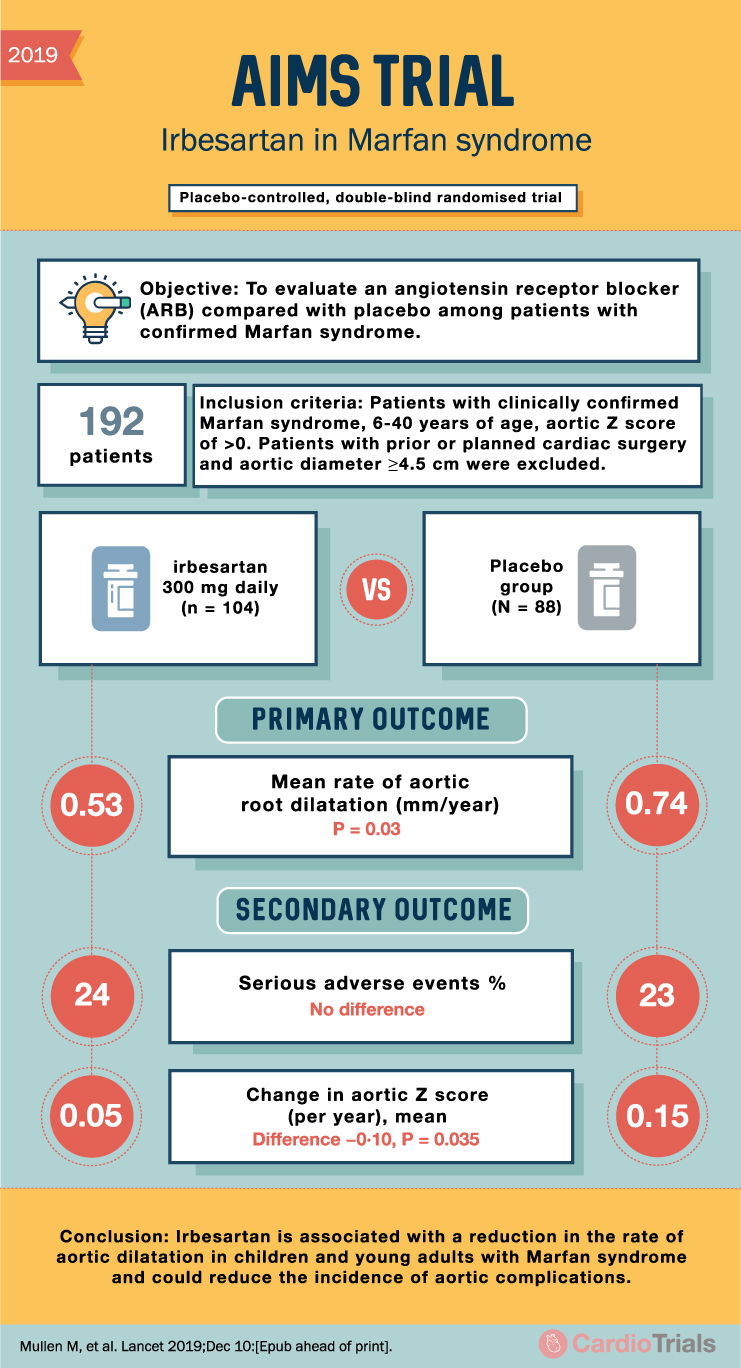
2019 AIMS TRIAL Irbesartan in Marfan syndrome Placebo-controlled, double-blind randomised trial Objective: To evaluate an angiotensin receptor blocker (ARB) compared with placebo among patients with confirmed Marfan syndrome. 192 patients Inclusion criteria: Patients with clinically confirmed Marfan syndrome, 6-40 years of age, aortic Z score of >0. Patients with prior or planned cardiac surgery and aortic diameter ≥4.5 cm were excluded. 0.53 irbesartan 300 mg daily (n = 104) VS Placebo group (N = 88) 贡 PRIMARY OUTCOME Mean rate of aortic root dilatation (mm/year) P = 0.03 SECONDARY OUTCOME 0.74 24 Serious adverse events % No difference 23 Change in aortic Z score 0.05 (per year), mean Difference -0.10, P = 0.035 0.15 Conclusion: Irbesartan is associated with a reduction in the rate of aortic dilatation in children and young adults with Marfan syndrome and could reduce the incidence of aortic complications. Mullen M, et al. Lancet 2019;Dec 10:[Epub ahead of print].