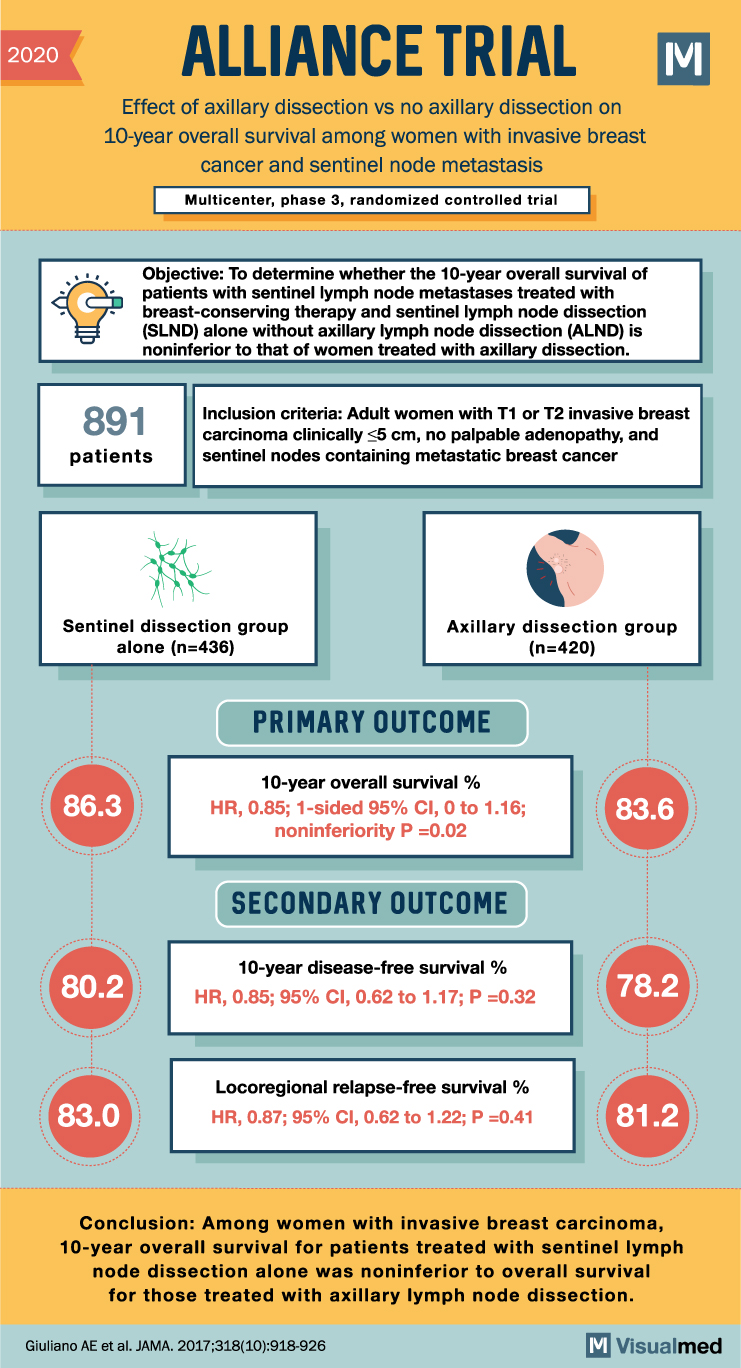
2020 ALLIANCE TRIAL Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis Multicenter, phase 3, randomized controlled trial M Objective: To determine whether the 10-year overall survival of patients with sentinel lymph node metastases treated with breast-conserving therapy and sentinel lymph node dissection (SLND) alone without axillary lymph node dissection (ALND) is noninferior to that of women treated with axillary dissection. 891 patients Inclusion criteria: Adult women with T1 or T2 invasive breast carcinoma clinically ≤5 cm, no palpable adenopathy, and sentinel nodes containing metastatic breast cancer Sentinel dissection group alone (n=436) Axillary dissection group (n=420) PRIMARY OUTCOME 10-year overall survival % 86.3 HR, 0.85; 1-sided 95% CI, 0 to 1.16; noninferiority P=0.02 83.6 SECONDARY OUTCOME 10-year disease-free survival % 80.2 HR, 0.85; 95% CI, 0.62 to 1.17; P=0.32 78.2 Locoregional relapse-free survival % 83.0 HR, 0.87; 95% CI, 0.62 to 1.22; P=0.41 81.2 Conclusion: Among women with invasive breast carcinoma, 10-year overall survival for patients treated with sentinel lymph node dissection alone was noninferior to overall survival for those treated with axillary lymph node dissection. Giuliano AE et al. JAMA. 2017;318(10):918-926