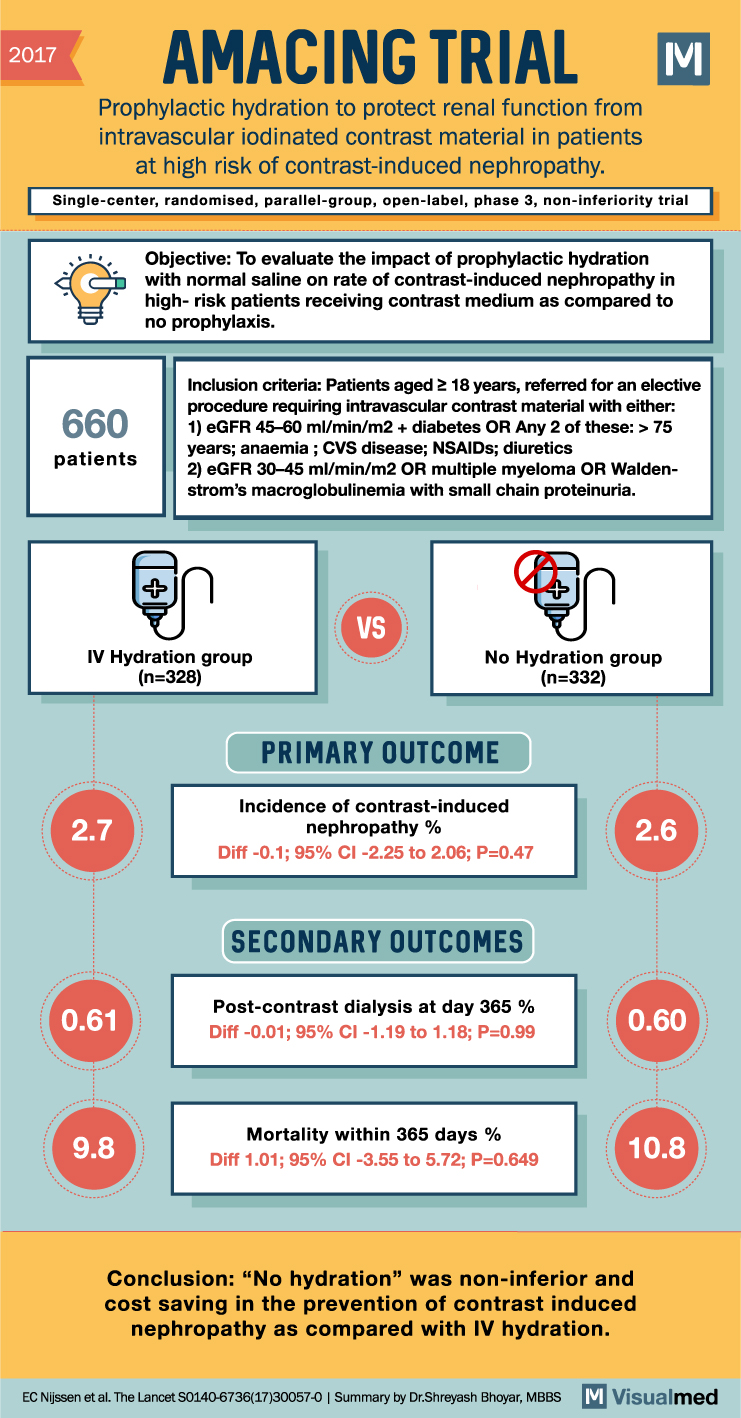
2017 AMACING TRIAL Prophylactic hydration to protect renal function from intravascular iodinated contrast material in patients at high risk of contrast-induced nephropathy. M Single-center, randomised, parallel-group, open-label, phase 3, non-inferiority trial 660 patients Objective: To evaluate the impact of prophylactic hydration with normal saline on rate of contrast-induced nephropathy in high-risk patients receiving contrast medium as compared to no prophylaxis. Inclusion criteria: Patients aged ≥ 18 years, referred for an elective procedure requiring intravascular contrast material with either: 1) eGFR 45-60 ml/min/m2 + diabetes OR Any 2 of these: > 75 years; anaemia; CVS disease; NSAIDs; diuretics 2) eGFR 30-45 ml/min/m2 OR multiple myeloma OR Walden- strom’s macroglobulinemia with small chain proteinuria. IV Hydration group (n=328) 2.7 VS No Hydration group (n=332) PRIMARY OUTCOME Incidence of contrast-induced nephropathy % Diff -0.1; 95% CI -2.25 to 2.06; P=0.47 SECONDARY OUTCOMES 2.6 0.61 Post-contrast dialysis at day 365 % Diff -0.01; 95% CI -1.19 to 1.18; P=0.99 0.60 Mortality within 365 days % 9.8 10.8 Diff 1.01; 95% CI -3.55 to 5.72; P=0.649 Conclusion: “No hydration” was non-inferior and cost saving in the prevention of contrast induced nephropathy as compared with IV hydration. EC Nijssen et al. The Lancet S0140-6736(17)30057-0