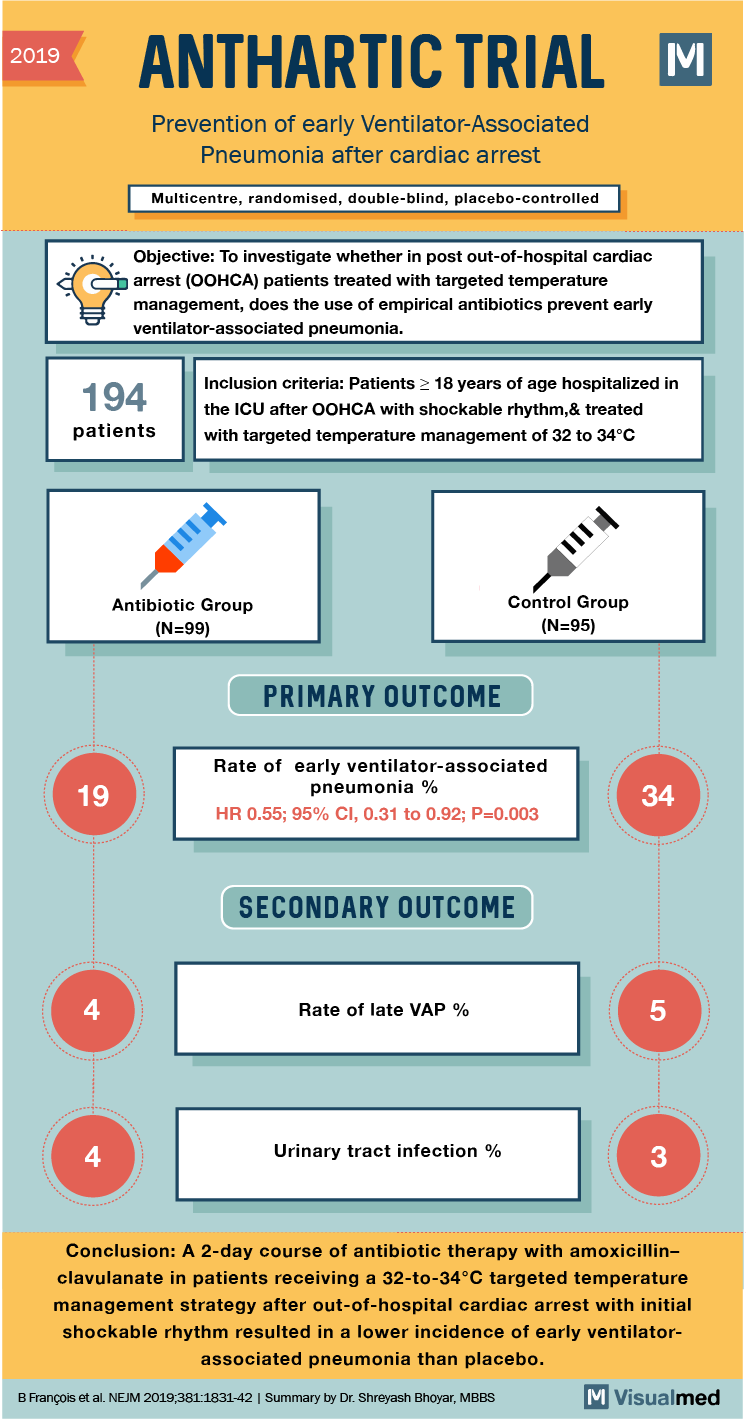
2019 ANTHARTIC TRIAL M Prevention of early Ventilator-Associated Pneumonia after cardiac arrest Multicentre, randomised, double-blind, placebo-controlled Objective: To investigate whether in post out-of-hospital cardiac arrest (OOHCA) patients treated with targeted temperature management, does the use of empirical antibiotics prevent early ventilator-associated pneumonia. 194 patients Inclusion criteria: Patients > 18 years of age hospitalized in the ICU after OOHCA with shockable rhythm,& treated with targeted temperature management of 32 to 34°C Antibiotic Group (N=99) Control Group (N=95) PRIMARY OUTCOME Rate of early ventilator-associated pneumonia % HR 0.55; 95% CI, 0.31 to 0.92; P=0.003 SECONDARY OUTCOME Rate of late VAP % Urinary tract infection % Conclusion: A 2-day course of antibiotic therapy with amoxicillinclavulanate in patients receiving a 32-to-34°C targeted temperature management strategy after out-of-hospital cardiac arrest with initial shockable rhythm resulted in a lower incidence of early ventilator associated pneumonia than placebo. B François et al. NEJM 2019,381:1831-42