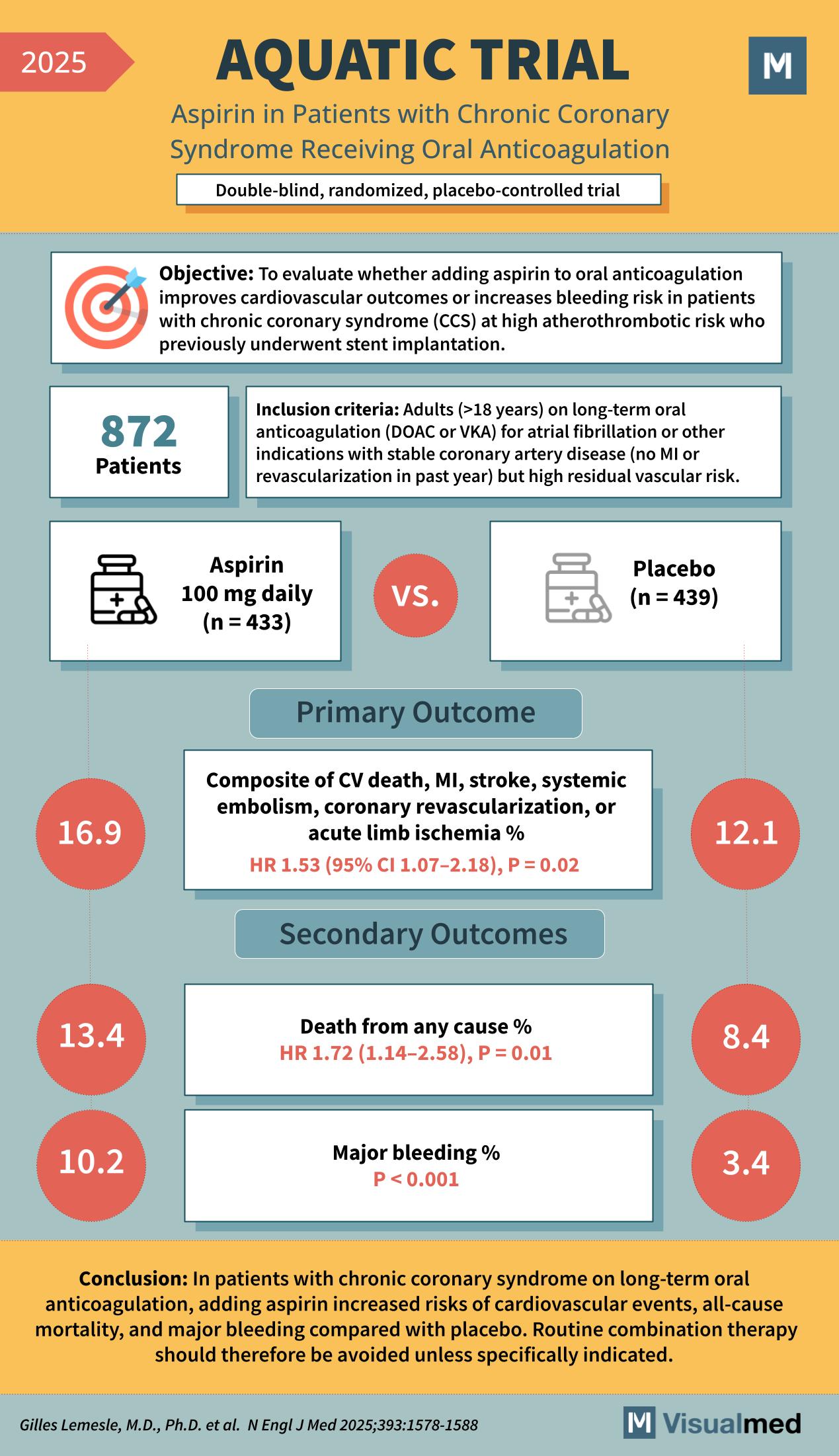
The AQUATIC trial (2025) was a double-blind, randomized, placebo-controlled study that evaluated the effect of adding aspirin to long-term oral anticoagulation in patients with chronic coronary syndrome (CCS) at high atherothrombotic risk. A total of 872 patients on oral anticoagulation (DOAC or VKA) for atrial fibrillation or other indications and with stable coronary artery disease were randomized to aspirin 100 mg daily or placebo. The primary outcome, a composite of cardiovascular death, myocardial infarction, stroke, systemic embolism, coronary revascularization, or acute limb ischemia, occurred more often with aspirin (16.9%) than with placebo (12.1%) — HR 1.53, P = 0.02. Aspirin was also linked to higher rates of death from any cause (13.4% vs. 8.4%, HR 1.72, P = 0.01) and major bleeding (10.2% vs. 3.4%, P < 0.001). The study concluded that in CCS patients on oral anticoagulants, adding aspirin increased risks of cardiovascular events, mortality, and bleeding, and should be avoided unless clearly indicated.
Source