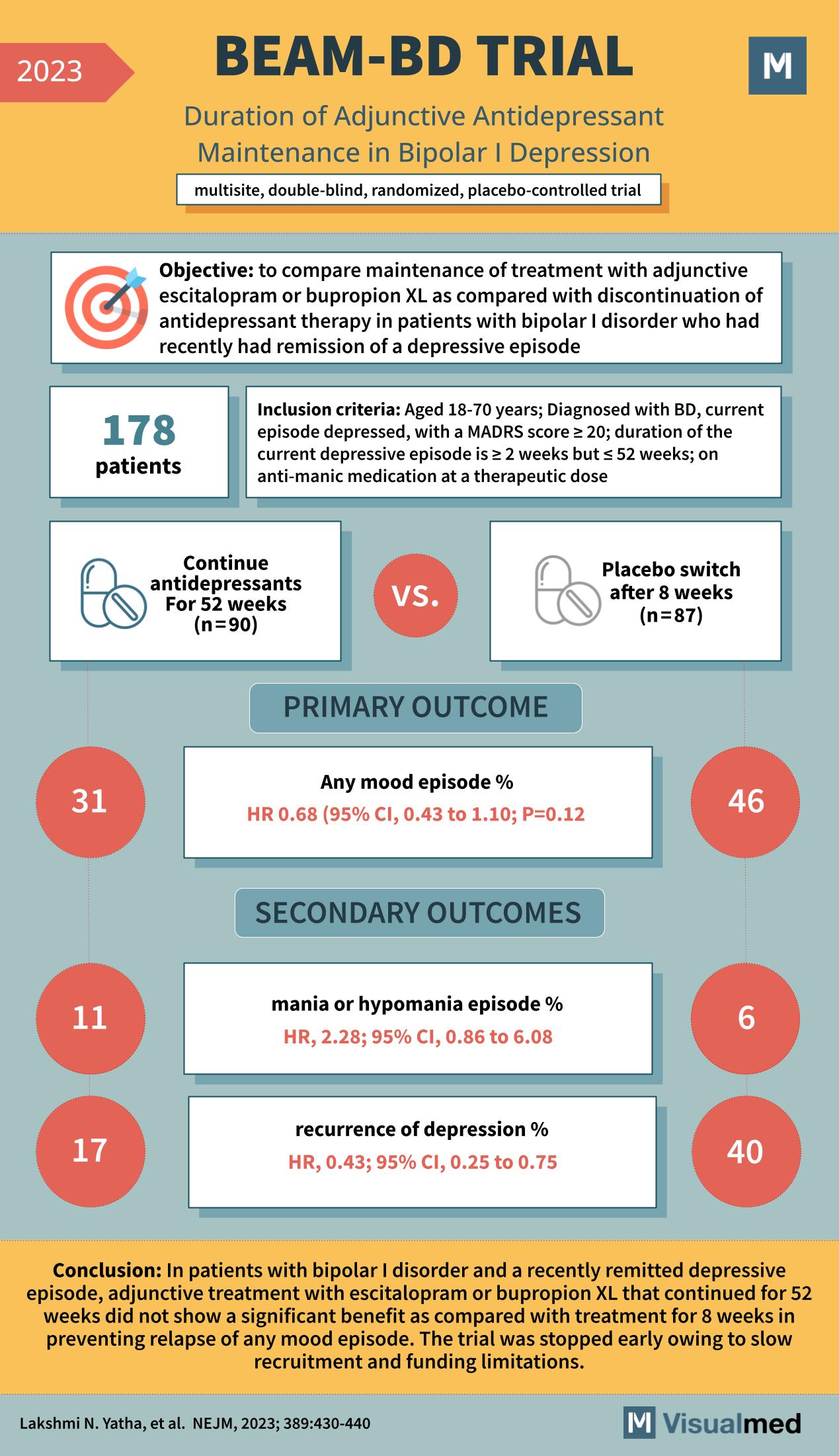
The BEAM-BD trial, as presented in the New England Journal of Medicine in 2023, was a multisite, double-blind, randomized, placebo-controlled trial with the objective of comparing the maintenance of treatment with adjunctive escitalopram or bupropion XL versus discontinuation of antidepressant therapy in patients with bipolar I disorder who had recently had remission of a depressive episode.
The study included 178 patients who met the inclusion criteria, including a diagnosis of bipolar disorder (BD), a current depressive episode, and an established score on the Montgomery-Åsberg Depression Rating Scale (MADRS). The participants were assigned to either continue with antidepressant treatment for 52 weeks (n=90) or to switch to a placebo after 8 weeks (n=87).
The primary outcome was the percentage of patients experiencing any mood episode. The results showed 31% in the continued antidepressant group versus 46% in the placebo group, with a hazard ratio (HR) of 0.68, indicating a trend favoring the continuation of antidepressants, although this did not reach statistical significance (95% CI, 0.43 to 1.10; P=0.12).
Secondary outcomes included the percentage of patients experiencing mania or hypomania episodes and the recurrence of depression. The incidence of mania or hypomania was higher in the continued antidepressant group (11%) compared to the placebo group (6%), with an HR of 2.28. However, the continued treatment group had a lower percentage of depression recurrence (17%) compared to the placebo group (40%), with an HR of 0.43, indicating a significant benefit in preventing depressive episodes.
The conclusion of the BEAM-BD trial was that in patients with bipolar I disorder and a recently remitted depressive episode, continuing treatment with escitalopram or bupropion XL for 52 weeks did not show a significant benefit compared to treatment for 8 weeks in preventing relapse of any mood episode. However, the continuation of antidepressants did show a benefit in reducing the recurrence of depressive episodes. The trial was stopped early due to slow recruitment and funding limitations, which may have affected the ability to detect a significant difference in the primary outcome. The BEAM-BD trial provides valuable insights into the management of bipolar I disorder, suggesting that while extended antidepressant treatment may help prevent depressive relapses, it does not significantly change the overall rate of mood episodes when compared to a shorter duration of treatment.