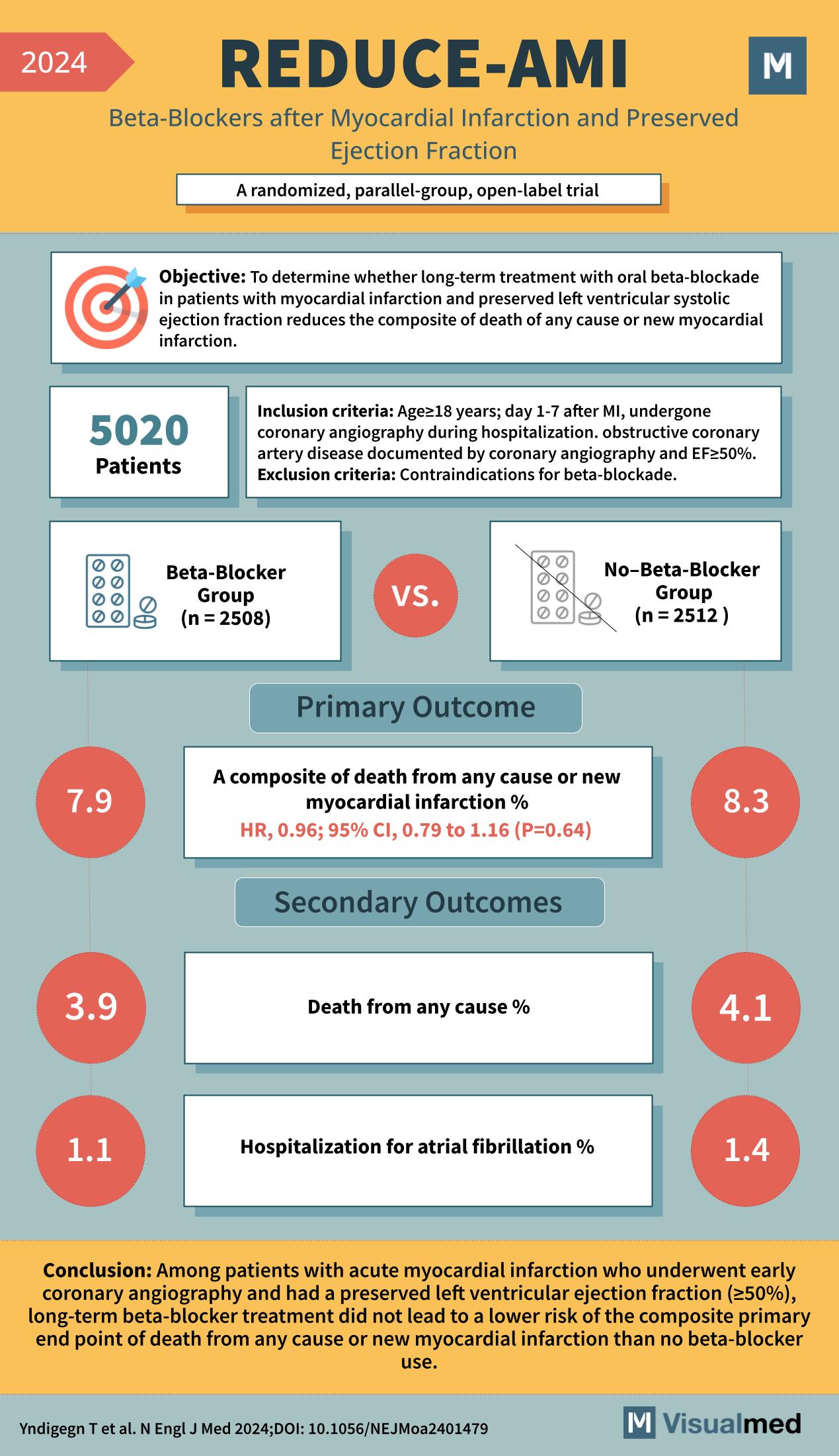
Year: 2024 Title: REDUCE-AMI Subtitle: Beta-Blockers after Myocardial Infarction and Preserved Ejection Fraction Type of Trial: A randomized, parallel-group, open-label trial
Objective: To determine whether long-term treatment with oral beta-blockade in patients with myocardial infarction and preserved left ventricular systolic ejection fraction reduces the composite of death of any cause or new myocardial infarction.
Patients: 5020
Inclusion Criteria:
- Age ≥18 years
- Day 1-7 after MI
- Undergone coronary angiography during hospitalization
- Obstructive coronary artery disease documented by coronary angiography and EF≥50%
Exclusion Criteria: Contraindications for beta-blockade.
Groups:
- Beta-Blocker Group (n = 2508)
- No-Beta-Blocker Group (n = 2512)
Primary Outcome:
- A composite of death from any cause or new myocardial infarction %
- HR, 0.96; 95% CI, 0.79 to 1.16 (P=0.64)
- Beta-Blocker Group: 7.9%
- No-Beta-Blocker Group: 8.3%
Secondary Outcomes:
- Death from any cause %
- Beta-Blocker Group: 3.9%
- No-Beta-Blocker Group: 4.1%
- Hospitalization for atrial fibrillation %
- Beta-Blocker Group: 1.1%
- No-Beta-Blocker Group: 1.4%
Conclusion: Among patients with acute myocardial infarction who underwent early coronary angiography and had a preserved left ventricular ejection fraction (≥50%), long-term beta-blocker treatment did not lead to a lower risk of the composite primary endpoint of death from any cause or new myocardial infarction than no beta-blocker use.
Reference: Yndigegn T et al. N Engl J Med 2024; DOI: 10.1056/NEJMoa2401479