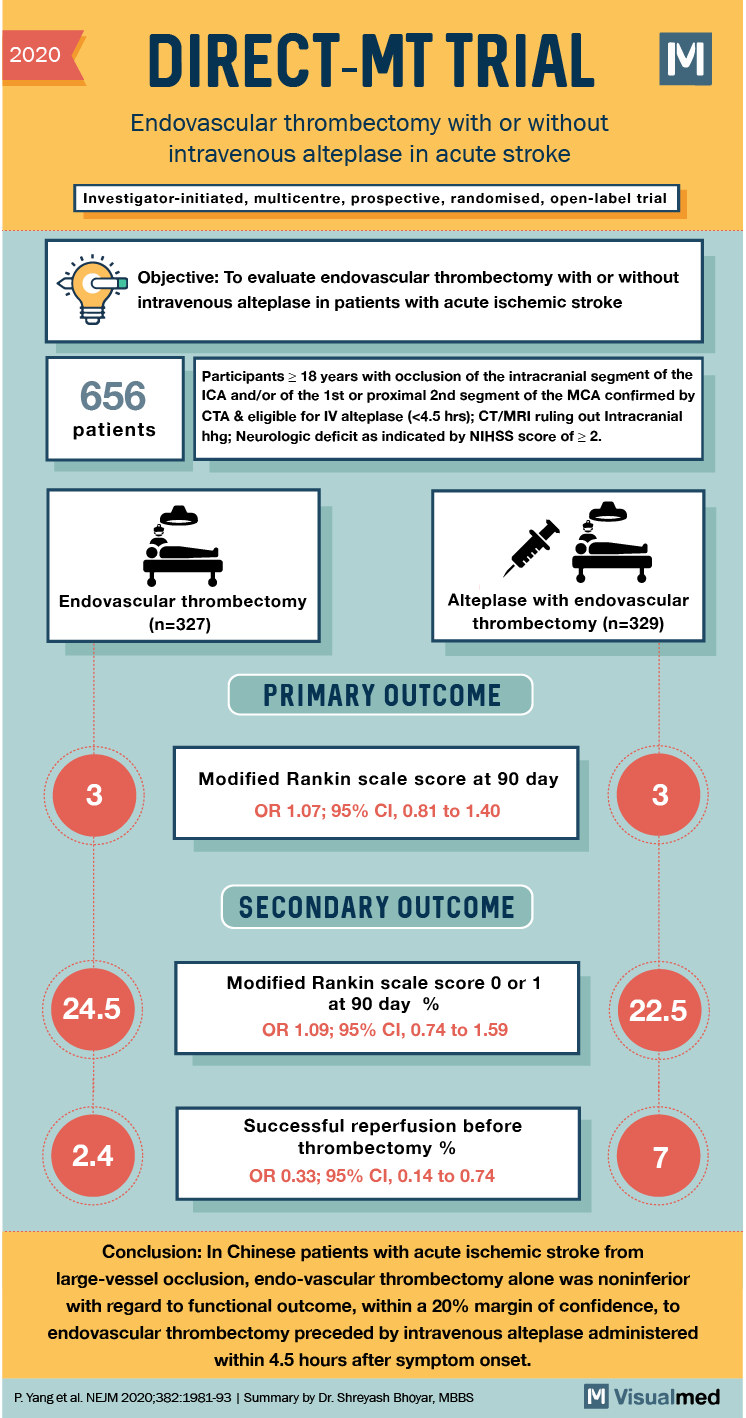
2020 DIRECT-MT TRIAL: Endovascular thrombectomy with or without intravenous alteplase in acute stroke Investigator-initiated, multicentre, prospective, randomised, open-label trial Objective: To evaluate endovascular thrombectomy with or without intravenous alteplase in patients with acute ischemic stroke 656 patients Participants > 18 years with occlusion of the intracranial segment of the ICA and/or of the 1st or proximal 2nd segment of the MCA confirmed by CTA & eligible for IV alteplase (<4.5 hrs); CT/MRI ruling out Intracranial hhg; Neurologic deficit as indicated by NIHSS score of 2. Endovascular thrombectomy (n=327) Alteplase with endovascular thrombectomy (n=329) ( PRIMARY OUTCOME Modified Rankin scale score at 90 day OR 1.07; 95% CI, 0.81 to 1.40 SECONDARY OUTCOME 24.5 Modified Rankin scale score 0 or 1 at 90 day % OR 1.09; 95% CI, 0.74 to 1.59 22.5 2.4 Successful reperfusion before thrombectomy % OR 0.33; 95% CI, 0.14 to 0.74 Conclusion: In Chinese patients with acute ischemic stroke from large-vessel occlusion, endo-vascular thrombectomy alone was noninferior with regard to functional outcome, within a 20% margin of confidence, to endovascular thrombectomy preceded by intravenous alteplase administered within 4.5 hours after symptom onset. P. Yang et al. NEJM 2020,382:1981-93 Summary by Dr. Shreyash Bhoyar, MBBS