The DUPLEX Trial: Evaluating Kidney Protection in FSGS Patients
The DUPLEX trial, a rigorous study published in the New England Journal of Medicine in 2023, has garnered significant attention in the nephrology community. The trial investigated the long-term nephroprotective potential of sparsentan versus irbesartan in patients with Focal Segmental Glomerulosclerosis (FSGS), a disease characterized by scarring in the kidney’s filtering units.
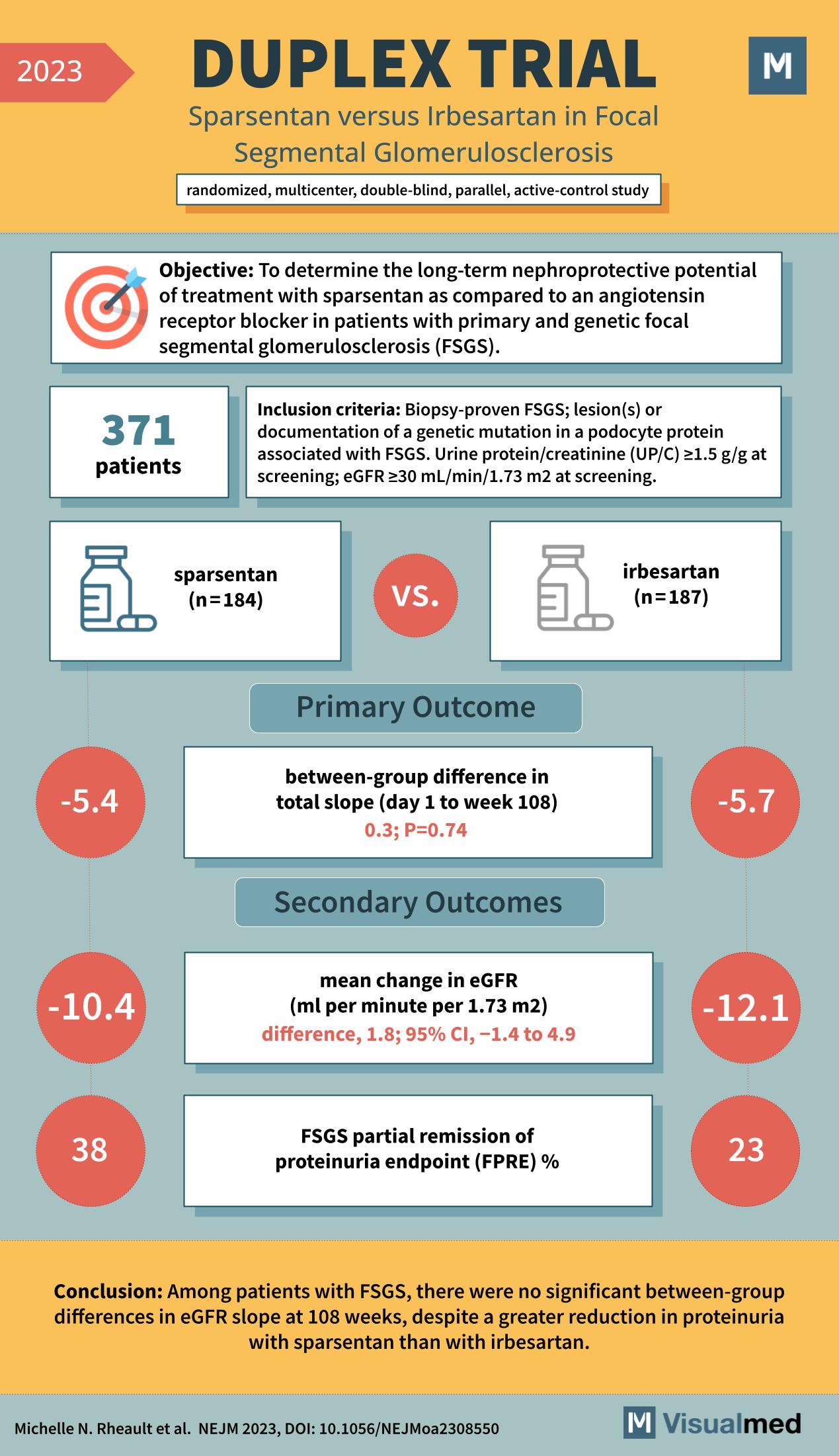
Trial Objectives and Design
The main aim of the DUPLEX trial was to determine the efficacy of sparsentan, a treatment option for patients with primary and genetic FSGS. The study was a randomized, multicenter, double-blind, parallel, active-control study, ensuring the highest levels of scientific rigor and credibility.
A total of 371 patients were enrolled based on strict inclusion criteria, including biopsy-proven FSGS, proteinuria, and a specific level of glomerular filtration rate (eGFR). The participants were divided into two groups: sparsentan (n=184) and irbesartan (n=187).
Results Overview
The primary outcome focused on the between-group difference in the total slope of eGFR from day 1 to week 108. Secondary outcomes included the mean change in eGFR and the percentage of patients achieving partial remission of proteinuria (FPRP).
In the primary outcome, the difference in the eGFR slope between the two treatments was minimal, with a statistical P value of 0.74, indicating no significant difference. However, the secondary outcomes presented a more nuanced picture. The mean change in eGFR was -10.4 mL/min/1.73 m^2 for sparsentan compared to -12.1 mL/min/1.73 m^2 for irbesartan. Furthermore, 38% of the sparsentan group reached the FPRP compared to 23% in the irbesartan group.
Conclusions and Clinical Impact
The DUPLEX trial concluded that, despite a lack of significant differences in eGFR slope at 108 weeks, there was a greater reduction in proteinuria with sparsentan compared to irbesartan. This suggests that while sparsentan might not significantly alter the progression of kidney function decline, it may offer better proteinuria control, a marker of kidney damage.
Implications for Future Research
While the DUPLEX trial’s results may not have established a clear superiority of sparsentan over irbesartan in terms of eGFR slope, the findings on proteinuria are promising and warrant further investigation. The trial underscores the importance of personalized medicine and the need for additional research to fully understand the potential benefits of sparsentan for patients with FSGS.
Conclusion
The DUPLEX trial stands as an important contribution to nephrology, particularly in the management of FSGS. It highlights the complexity of treating kidney diseases and the need for continued exploration of treatment options to improve patient outcomes.