ELEKT-D Trial: Ketamine vs. ECT for Treatment-Resistant Major Depression
Introduction:
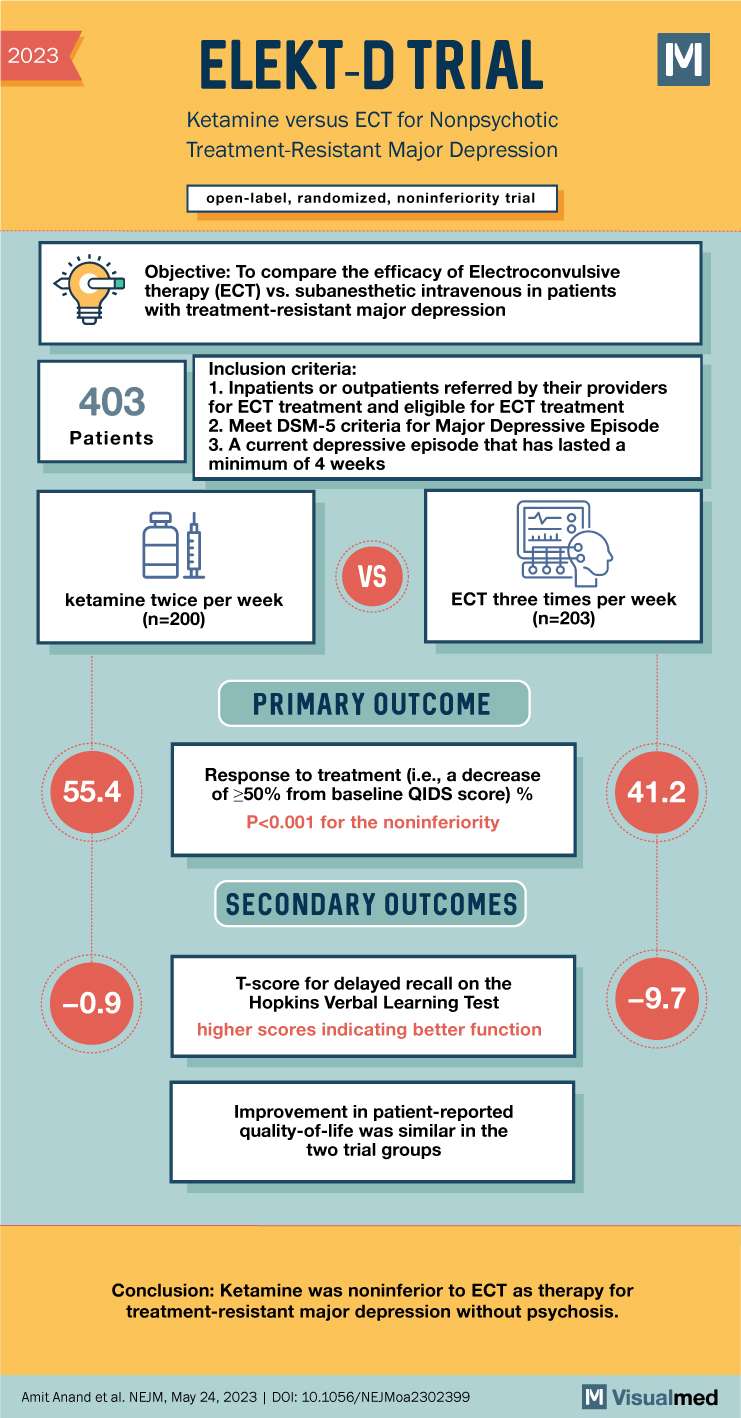
The groundbreaking ELEKT-D Trial sought to compare the efficacy of electroconvulsive therapy (ECT) and subanesthetic intravenous ketamine for the treatment of patients with treatment-resistant major depression. This study aimed to determine whether ketamine was noninferior to ECT in producing a treatment response and explored secondary outcomes such as memory function and patient-reported quality of life.
Methods:
The trial employed an open-label, randomized, noninferiority design, enrolling 403 patients diagnosed with treatment-resistant major depression without psychosis. Participants were assigned in a 1:1 ratio to receive either ketamine or ECT. Key features of the study methodology included:
- Initial Treatment Phase: Patients received ECT three times per week or ketamine (0.5 mg/kg over 40 minutes) twice per week over a period of three weeks.
- Primary Outcome Measure: Treatment response, defined as a ≥50% reduction in the score on the 16-item Quick Inventory of Depressive Symptomatology–Self-Report.
- Secondary Outcomes: Assessment of memory function through memory tests and evaluation of patient-reported quality of life.
- Follow-up Period: Patients who responded to treatment were followed for six months.
Results:
The trial outcomes revealed compelling findings, demonstrating the comparative effectiveness of ketamine and ECT in the treatment of major depression:
- Treatment Response: A significant response was observed in 55.4% of the ketamine group compared to 41.2% of the ECT group (difference of 14.2 percentage points; 95% confidence interval, 3.9 to 24.2; P<0.001), indicating that ketamine was noninferior to ECT.
- Memory Function: ECT treatment appeared to cause transient memory recall impairment during the initial phase, which showed gradual recovery during follow-up. In contrast, the ketamine group did not exhibit significant memory-related side effects.
- Patient-Reported Quality of Life: Both ketamine and ECT showed comparable improvements in patient-reported quality of life, indicating that both treatments had a positive impact on overall well-being.
- Adverse Effects: ECT was associated with musculoskeletal adverse effects, whereas ketamine was primarily associated with dissociative effects.
Conclusion:
The ELEKT-D Trial conclusively demonstrated that ketamine is noninferior to ECT for the treatment of treatment-resistant major depression without psychosis. These findings offer clinicians and patients an alternative therapeutic option for individuals who have not responded favorably to traditional treatments. The trial contributes valuable evidence to guide clinical decision-making in the management of treatment-resistant major depression, expanding treatment possibilities and potentially enhancing patient outcomes.
Inclusion and Exclusion Criteria:
To be eligible for the trial, participants had to meet the following criteria:
Inclusion Criteria:
- Written informed consent prior to study-related procedures
- Inpatients or outpatients referred for ECT treatment, meeting eligibility criteria
- Males/females aged 21 to 75 years
- Diagnosis of Major Depressive Episode according to DSM-5 criteria, confirmed through clinician’s diagnostic evaluation and the MINI International Neuropsychiatric Interview
- Current depressive episode lasting a minimum of four weeks
- Specific scores on symptom rating scales at screening: Montgomery Asberg Depression Rating Scale (MADRS) >20, Young Mania Rating Scale (YMRS) ≤5, Montreal Cognitive Assessment (MoCA) ≥18
- At least two adequate trials of antidepressants or augmentation strategies in their lifetime
- Patient’s willingness and ability to comply with scheduled visits, treatment plan, and trial procedures for the study’s duration
Exclusion Criteria:
- Diagnosis of bipolar disorder, schizophrenia, schizophreniform disorder, schizoaffective disorder, mental retardation, or pervasive developmental disorder according to DSM-5 criteria
- Meeting any exclusion criteria for ECT or ketamine treatment as described in clinical guidelines or determined by the investigator
- Pregnancy or breastfeeding
- Severe medical illness or severe neurological disorder
- Known ketamine allergy or concomitant use of medications that may interact with ketamine
- Diagnosis of major depressive disorder with psychotic features during the current depressive episode
- Inability to provide informed consent
- Prior enrollment or randomization in the trial
In summary, the ELEKT-D Trial provides compelling evidence that ketamine is noninferior to ECT in treating treatment-resistant major depression without psychosis. These findings offer an innovative therapeutic option for individuals who have not responded adequately to traditional treatments, expanding the range of possibilities for managing this challenging condition.