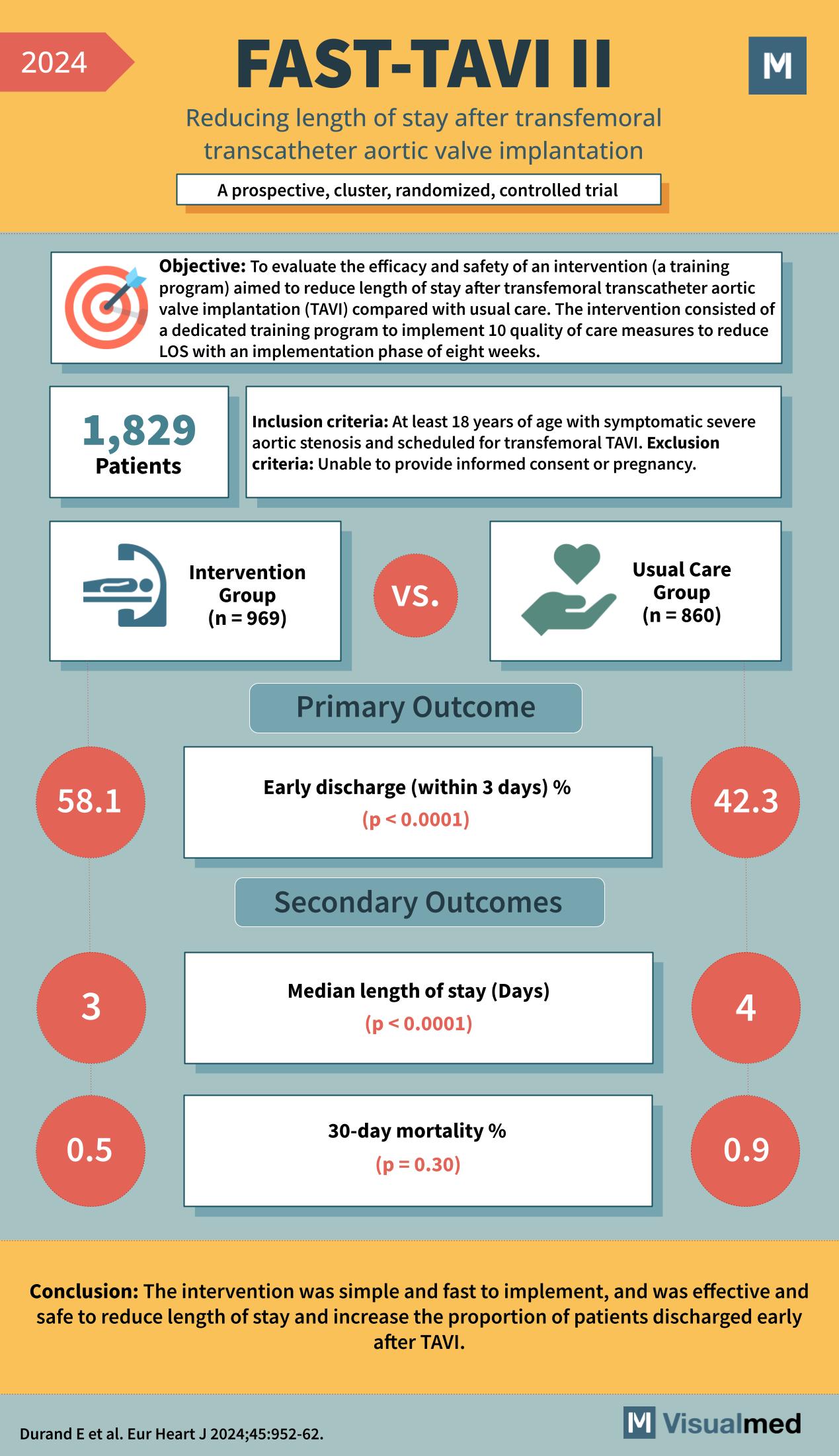
Year: 2024 Title: FAST-TAVI II Subtitle: Reducing length of stay after transfemoral transcatheter aortic valve implantation Type of Trial: A prospective, cluster, randomized, controlled trial
Objective: To evaluate the efficacy and safety of an intervention (a training program) aimed to reduce the length of stay after transfemoral transcatheter aortic valve implantation (TAVI) compared with usual care. The intervention consisted of a dedicated training program to implement 10 quality care measures to reduce LOS with an implementation phase of eight weeks.
Participants: 1,829
Inclusion Criteria: At least 18 years of age with symptomatic severe aortic stenosis and scheduled for transfemoral TAVI. Exclusion Criteria: Unable to provide informed consent or pregnancy.
Groups:
- Intervention Group (n = 969)
- Usual Care Group (n = 860)
Primary Outcome:
- Early discharge (within 3 days) %
- Intervention Group: 58.1% (p < 0.0001)
- Usual Care Group: 42.3%
Secondary Outcomes:
- Median length of stay (Days)
- Intervention Group: 3 days (p < 0.0001)
- Usual Care Group: 4 days
- 30-day mortality %
- Intervention Group: 0.5% (p = 0.30)
- Usual Care Group: 0.9%
Conclusion: The intervention was simple and fast to implement and was effective and safe to reduce the length of stay and increase the proportion of patients discharged early after TAVI.
Reference: Durand E et al. Eur Heart J 2024;45:952-62.