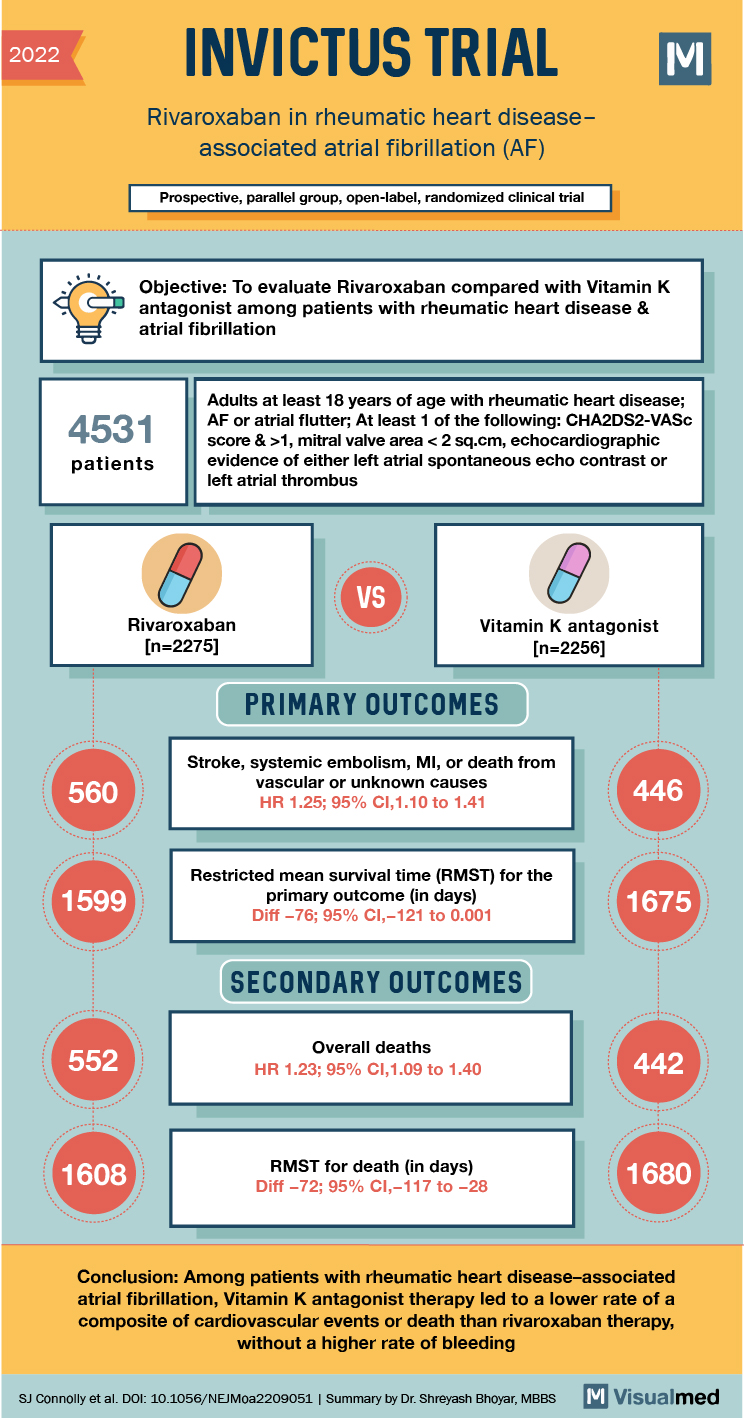
2022 INVICTUS TRIAL Rivaroxaban in rheumatic heart disease associated atrial fibrillation (AF) Prospective, parallel group, open-label, randomized clinical trial d e Objective: To evaluate Rivaroxaban compared with Vitamin K antagonist among patients with rheumatic heart disease & atrial fibrillation antagonis 4531 patients Adults at least 18 years of age with rheumatic heart disease; AF or atrial flutter; At least 1 of the following: CHA2DS2-VASC score & >1, mitral valve area < 2 sq.cm, echocardiographic evidence of either left atrial spontaneous echo contrast or left atrial thrombus Rivaroxaban
[n=2275]
Vitamin K antagonist (n=2256] In_ADEC PRIMARY OUTCOMES 560 Stroke, systemic embolism, MI, or death from vascular or unknown causes HR 1.25; 95% CI, 1.10 to 1.41 446 1599 Restricted mean survival time (RMST) for the primary outcome (in days) Diff -76; 95% CI,-121 to 0.001 1675 SECONDARY OUTCOMES 552 Overall deaths HR 1.23; 95% CI,1.09 to 1.40 442 1608 RMST for death (in days) Diff – 72; 95% CI,-117 to -28 1680 Conclusion: Among patients with rheumatic heart disease-associated atrial fibrillation, Vitamin K antagonist therapy led to a lower rate of a composite of cardiovascular events or death than rivaroxaban therapy, without a higher rate of bleeding SJ Connolly et al. Doi: 10.1056/NEJMoa2209051