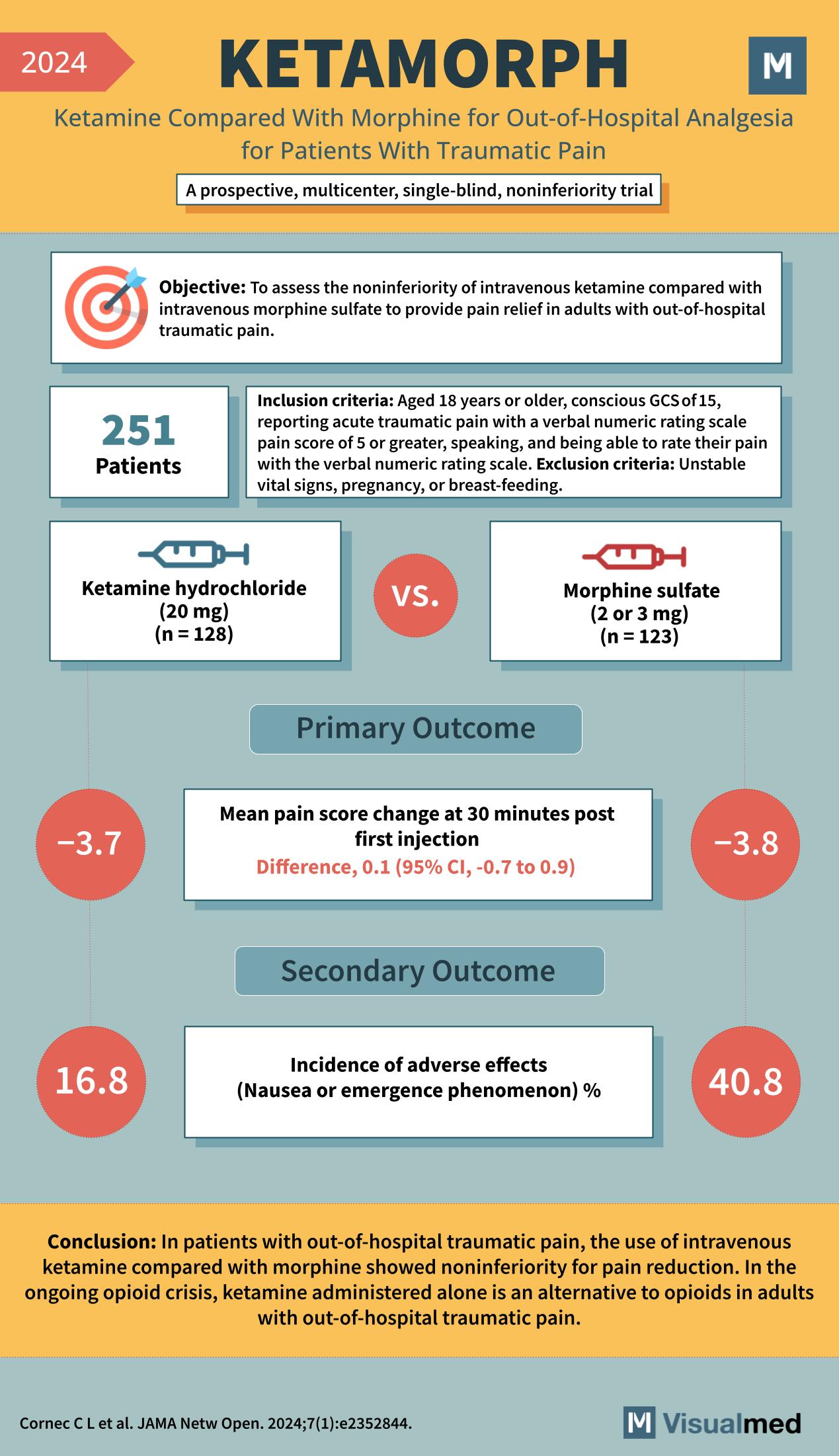
Year: 2024 Title: KETAMORPH Subtitle: Ketamine Compared With Morphine for Out-of-Hospital Analgesia for Patients With Traumatic Pain Type of Trial: A prospective, multicenter, single-blind, noninferiority trial
Objective: To assess the noninferiority of intravenous ketamine compared with intravenous morphine sulfate to provide pain relief in adults with out-of-hospital traumatic pain.
Participants: 251
Inclusion Criteria:
- Aged 18 years or older
- Conscious GCS of 15
- Reporting acute traumatic pain with a verbal numeric rating scale pain score of 5 or greater
- Speaking, and being able to rate their pain with the verbal numeric rating scale
Exclusion Criteria:
- Unstable vital signs
- Pregnancy
- Breast-feeding
Groups:
- Ketamine hydrochloride (20 mg) (n = 128)
- Morphine sulfate (2 or 3 mg) (n = 123)
Primary Outcome:
- Mean pain score change at 30 minutes post first injection
- Ketamine: -3.7
- Morphine: -3.8
- Difference, 0.1 (95% CI, -0.7 to 0.9)
Secondary Outcome:
- Incidence of adverse effects (Nausea or emergence phenomenon) %
- Ketamine: 16.8%
- Morphine: 40.8%
Conclusion: In patients with out-of-hospital traumatic pain, the use of intravenous ketamine compared with morphine showed noninferiority for pain reduction. In the ongoing opioid crisis, ketamine administered alone is an alternative to opioids in adults with out-of-hospital traumatic pain.
Reference: CorneC L et al. JAMA Netw Open. 2024;7(1):e2352844