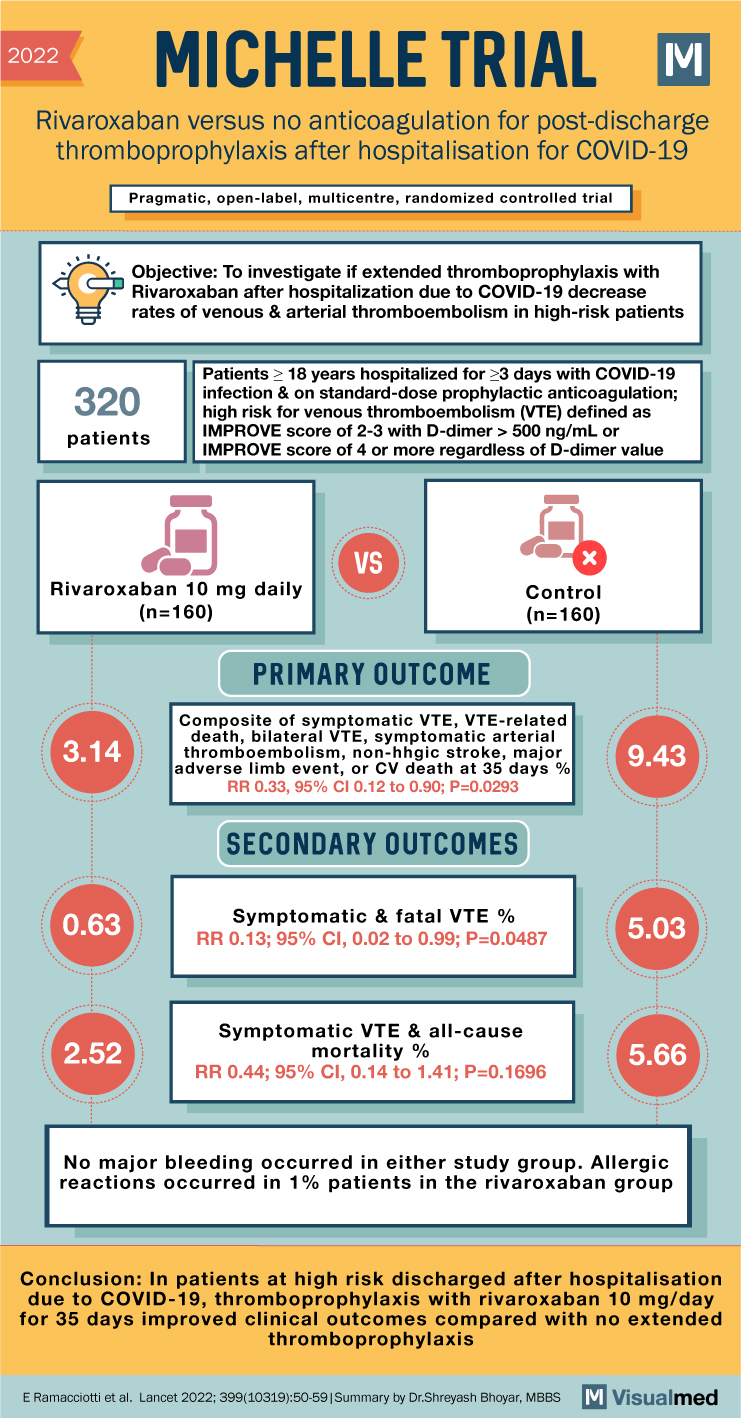
2022 MICHELLE TRIAL Rivaroxaban versus no anticoagulation for post-discharge thromboprophylaxis after hospitalisation for COVID-19 Pragmatic, open-label, multicentre, randomized controlled trial Objective: To investigate if extended thromboprophylaxis with Rivaroxaban after hospitalization due to COVID-19 decrease rates of venous & arterial thromboembolism in high-risk patients 320 patients Patients ≥ 18 years hospitalized for ≥3 days with COVID-19 infection & on standard-dose prophylactic anticoagulation; high risk for venous thromboembolism (VTE) defined as IMPROVE score of 2-3 with D-dimer > 500 ng/mL or IMPROVE score of 4 or more regardless of D-dimer value VS X Rivaroxaban 10 mg daily Control (n=160) (n=160) PRIMARY OUTCOME 3.14 Composite of symptomatic VTE, VTE-related death, bilateral VTE, symptomatic arterial thromboembolism, non-hhgic stroke, major adverse limb event, or CV death at 35 days% RR 0.33, 95% CI 0.12 to 0.90; P=0.0293 9.43 SECONDARY OUTCOMES 0.63 Symptomatic & fatal VTE % 5.03 RR 0.13; 95% CI, 0.02 to 0.99; P=0.0487 2.52 Symptomatic VTE & all-cause mortality % 5.66 RR 0.44; 95% CI, 0.14 to 1.41; P=0.1696 No major bleeding occurred in either study group. Allergic reactions occurred in 1% patients in the rivaroxaban group Conclusion: In patients at high risk discharged after hospitalisation due to COVID-19, thromboprophylaxis with rivaroxaban 10 mg/day for 35 days improved clinical outcomes compared with no extended thromboprophylaxis E Ramacciotti et al. Lancet 2022; 399(10319):50-59