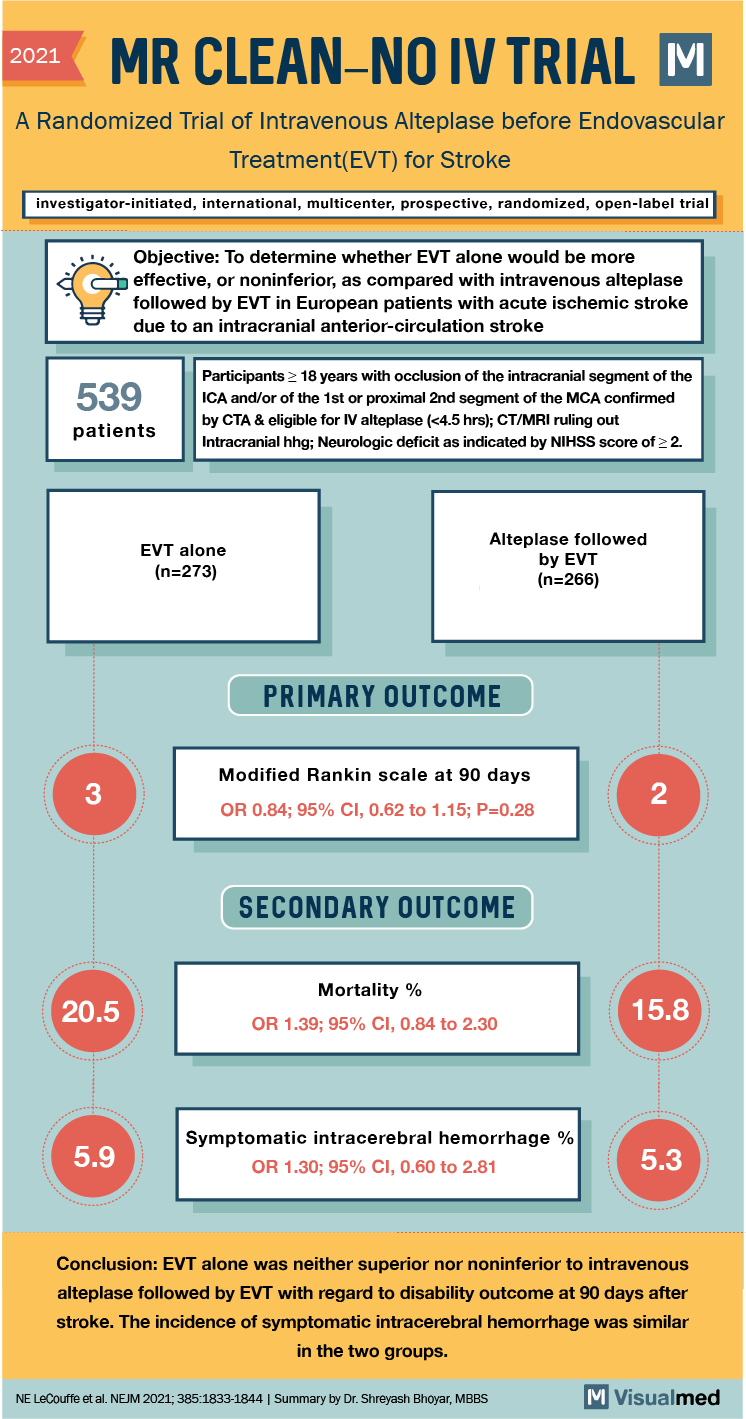
2021 MR CLEAN-NO IV TRIAL M 2021 A Randomized Trial of Intravenous Alteplase before Endovascular Treatment(EVT) for Stroke investigator-initiated, international, multicenter, prospective, randomized, open-label trial ” d Objective: To determine whether EVT alone would be more effective, or noninferior, as compared with intravenous alteplase followed by EVT in European patients with acute ischemic stroke due to an intracranial anterior-circulation stroke 539 Participants > 18 years with occlusion of the intracranial segment of the ICA and/or of the 1st or proximal 2nd segment of the MCA confirmed by CTA & eligible for IV alteplase (<4.5 hrs); CT/MRI ruling out Intracranial hhg; Neurologic deficit as indicated by NIHSS score of 2. patients EVT alone (n=273) Alteplase followed by EVT (n=266) PRIMARY OUTCOME Modified Rankin scale at 90 days OR 0.84; 95% CI, 0.62 to 1.15; P=0.28 SECONDARY OUTCOME 20.5 Mortality % OR 1.39; 95% CI, 0.84 to 2.30 15.8 5.9 Symptomatic intracerebral hemorrhage % OR 1.30; 95% CI, 0.60 to 2.81 5.3 Conclusion: EVT alone was neither superior nor noninferior to intravenous alteplase followed by EVT with regard to disability outcome at 90 days after stroke. The incidence of symptomatic intracerebral hemorrhage was similar in the two groups. NE LeCouffe et al. NEJM 2021;385:1833-1844