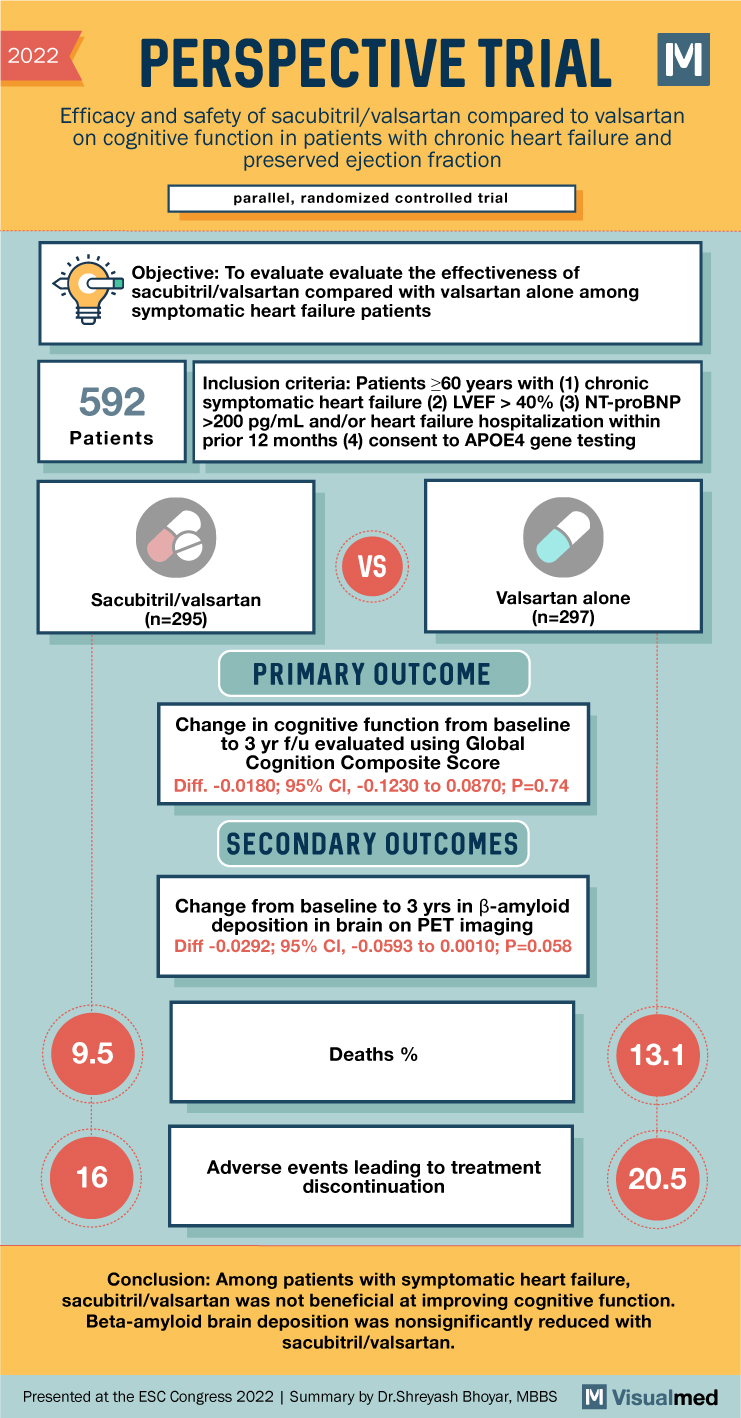
2022 PERSPECTIVE TRIAL M Efficacy and safety of sacubitril/valsartan compared to valsartan on cognitive function in patients with chronic heart failure and preserved ejection fraction parallel, randomized controlled trial Objective: To evaluate the effectiveness of sacubitril/valsartan compared with valsartan alone among symptomatic heart failure patients 592 Patients Inclusion criteria: Patients ≥60 years with (1) chronic symptomatic heart failure (2) LVEF > 40% (3) NT-proBNP >200 pg/mL and/or heart failure hospitalization within prior 12 months (4) consent to APOE4 gene testing Sacubitril/valsartan (n=295) VS Valsartan alone (n=297) PRIMARY OUTCOME Change in cognitive function from baseline to 3 yr f/u evaluated using Global Cognition Composite Score Diff. -0.0180; 95% CI, -0.1230 to 0.0870; P=0.74 SECONDARY OUTCOMES Change from baseline to 3 yrs in ẞ-amyloid deposition in brain on PET imaging Diff -0.0292; 95% CI, -0.0593 to 0.0010; P=0.058 9.5 166 Deaths % Adverse events leading to treatment discontinuation 13.1 20.5 Conclusion: Among patients with symptomatic heart failure, sacubitril/valsartan was not beneficial at improving cognitive function. Beta-amyloid brain deposition was nonsignificantly reduced with sacubitril/valsartan. Presented at the ESC Congress 2022