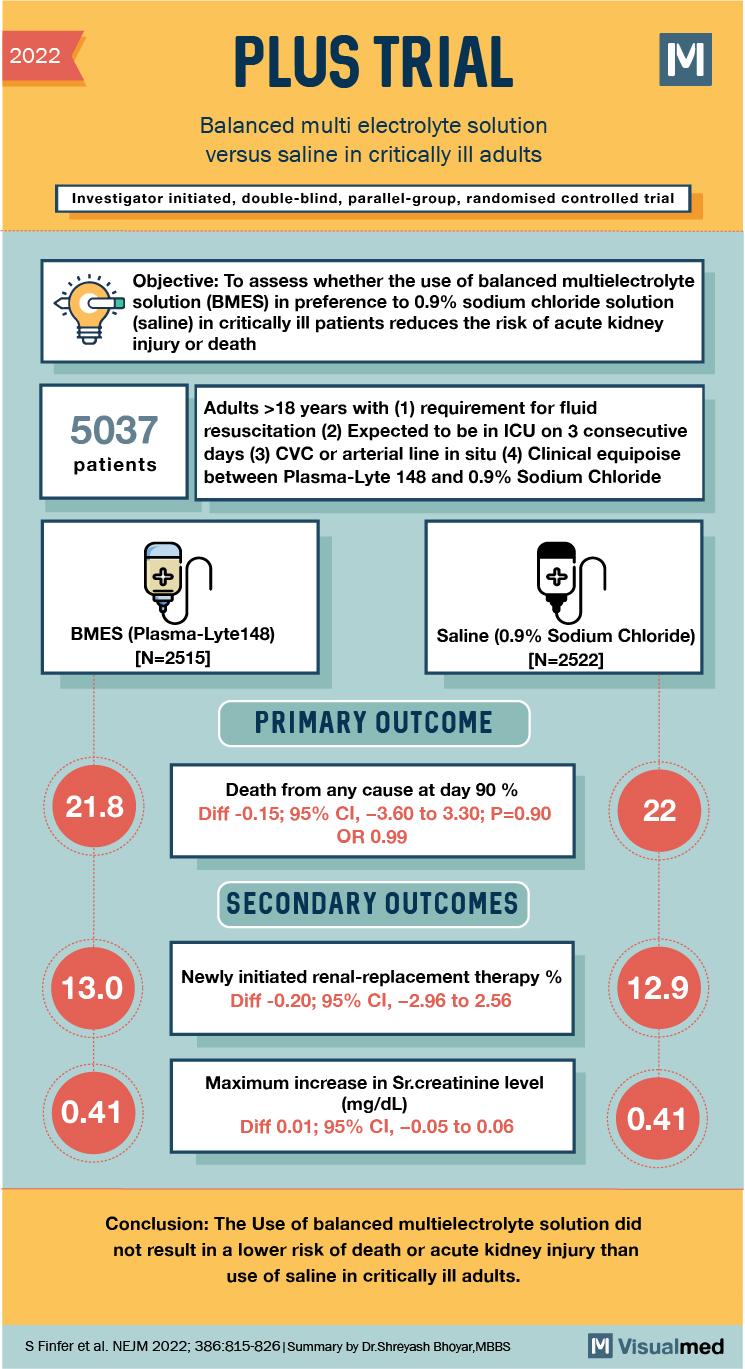
2022 PLUS TRIAL Balanced multi electrolyte solution versus saline in critically ill adults Investigator initiated, double-blind, parallel-group, randomised controlled trial d a Objective: To assess whether the use of balanced multielectrolyte solution (BMES) in preference to 0.9% sodium chloride solution (saline) in critically ill patients reduces the risk of acute kidney injury or death 5037 Adults >18 years with (1) requirement for fluid resuscitation (2) Expected to be in ICU on 3 consecutive days (3) CVC or arterial line in situ (4) Clinical equipoise between Plasma-Lyte 148 and 0.9% Sodium Chloride patients BMES (Plasma-Lyte148) [N=2515] Saline (0.9% Sodium Chloride) [N=2522] PRIMARY OUTCOME 21.8 Death from any cause at day 90 % Diff -0.15; 95% CI, -3.60 to 3.30; P=0.90 OR 0.99 SECONDARY OUTCOMES 13.0 Newly initiated renal-replacement therapy % Diff -0.20; 95% CI, -2.96 to 2.56 12.9 0.41 Maximum increase in Sr.creatinine level (mg/dL) Diff 0.01; 95% CI, -0.05 to 0.06 0.41 Conclusion: The Use of balanced multielectrolyte solution did not result in a lower risk of death or acute kidney injury than use of saline in critically ill adults. S Finfer et al. NEJM 2022; 386:815-826