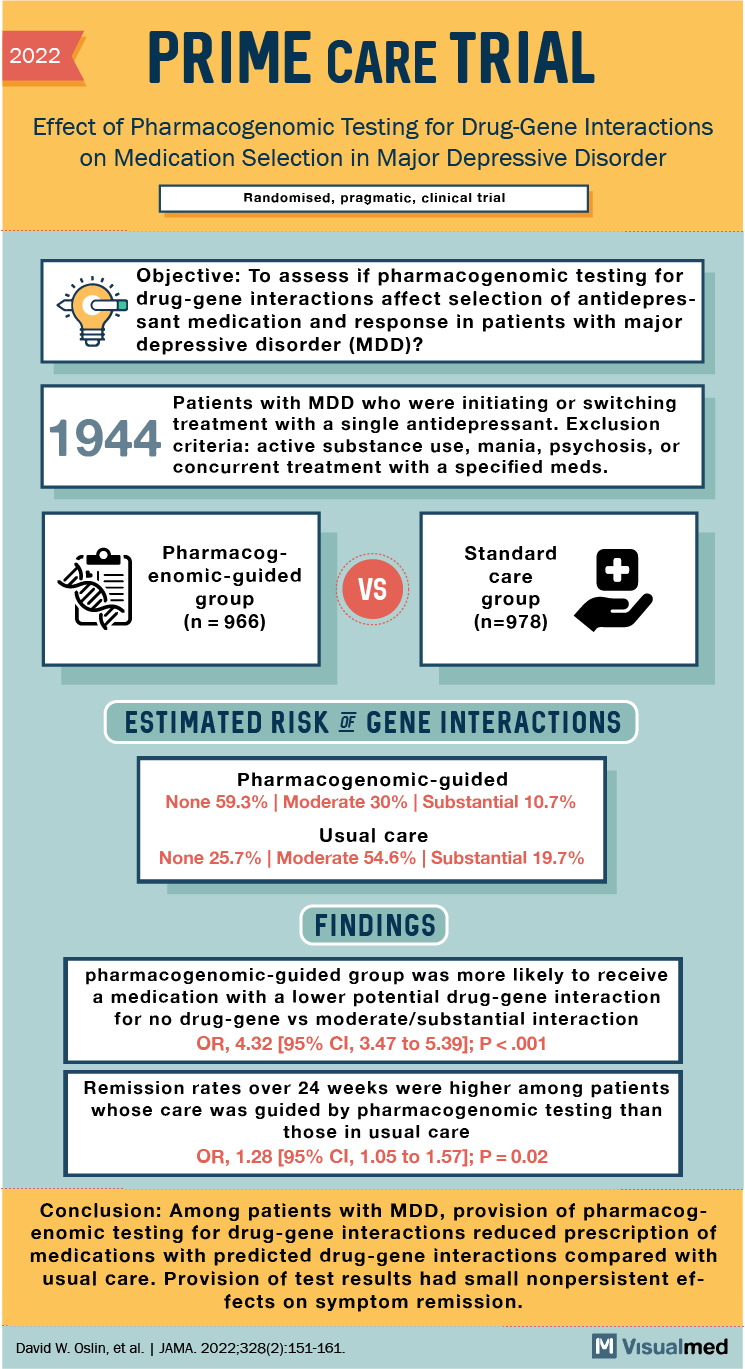
2022 PRIME CARE TRIAL Effect of Pharmacogenomic Testing for Drug-Gene Interactions on Medication Selection in Major Depressive Disorder Randomised, pragmatic, clinical trial Objective: To assess if pharmacogenomic testing for drug-gene interactions affects the selection of antidepressant medication and response in patients with major depressive disorder (MDD)? 4 Patients with MDD who were initiating or switching treatment with a single antidepressant. Exclusion criteria: active substance use, mania, psychosis, or concurrent treatment with specified meds. Pharmacogenomic-guided group (n = 966) VS Standard care group (n=978) ESTIMATED RISK OF GENE INTERACTIONS Pharmacogenomic-guided None 59.3% Moderate 30% Substantial 10.7% Usual care None 25.7% Moderate 54.6% Substantial 19.7% FINDINGS pharmacogenomic-guided group was more likely to receive a medication with a lower potential drug-gene interaction for no drug-gene vs moderate/substantial interaction OR, 4.32 [95% CI, 3.47 to 5.39]; P<.001 Remission rates over 24 weeks were higher among patients whose care was guided by pharmacogenomic testing than those in usual care OR, 1.28 [95% CI, 1.05 to 1.57]; P=0.02 Conclusion: Among patients with MDD, provision of pharmacogenomic testing for drug-gene interactions reduced prescription of medications with predicted drug-gene interactions compared with usual care. Provision of test results had small nonpersistent ef fects on symptom remission. David W. Oslin, et al. JAMA 2022;328(2):151-161. M Visualmed