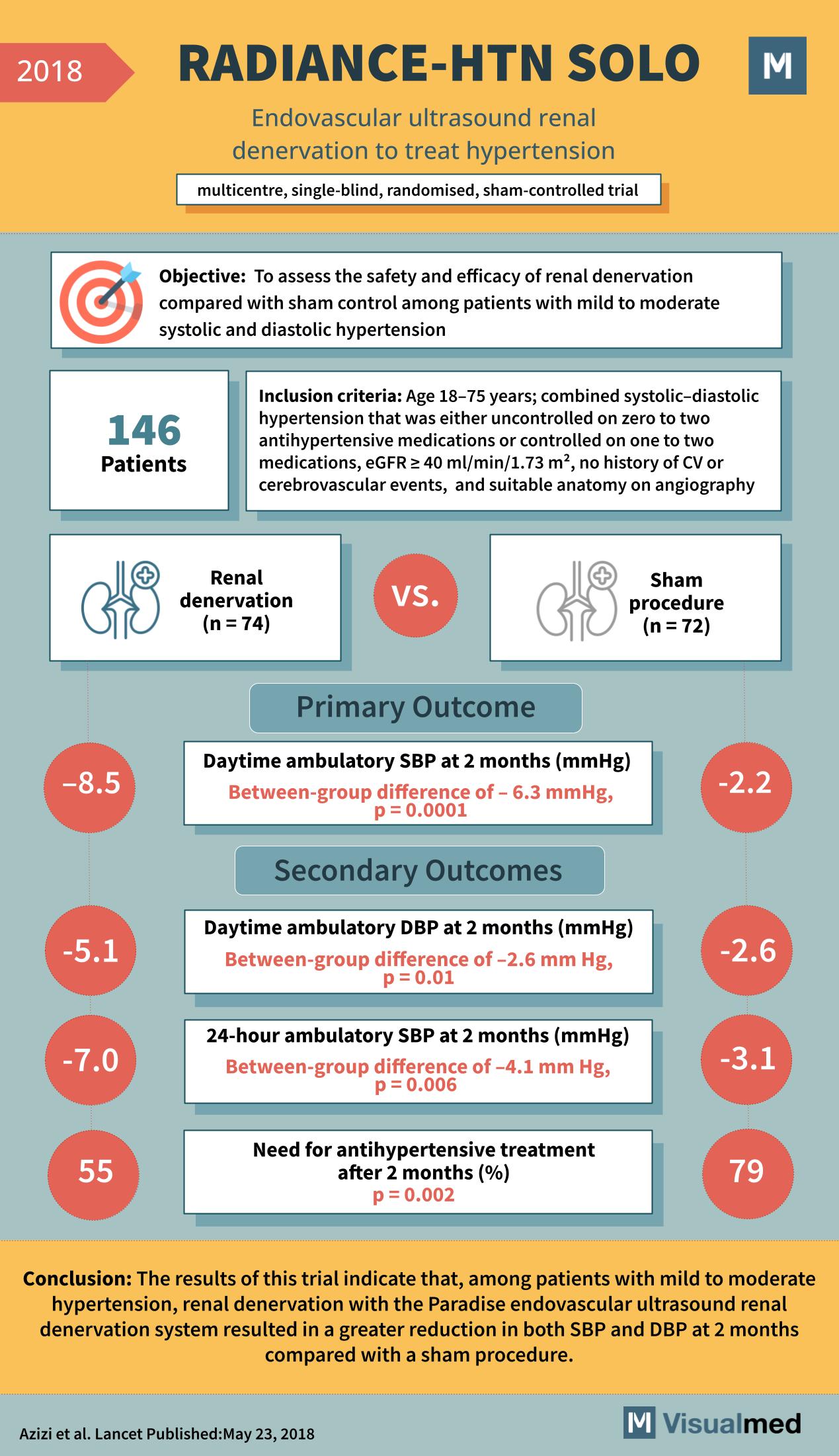
The RADIANCE-HTN SOLO trial, published in The Lancet in May 2018, marks a significant stride in hypertension treatment research. This trial focused on the safety and efficacy of a novel endovascular ultrasound renal denervation procedure compared to a sham procedure in patients with mild to moderate hypertension, reframing our approach to managing this pervasive cardiovascular risk factor.
The trial was a multicenter, single-blind, randomized, sham-controlled study that enrolled 146 patients with combined systolic-diastolic hypertension. These patients were either not on antihypertensive medications or were controlled on a maximum of two medications. They were required to have a stable estimated glomerular filtration rate (eGFR ≥ 40 ml/min/1.73 m²), no history of cardiovascular or cerebrovascular events, and suitable anatomy for the procedure as confirmed by angiography.
The primary outcome measured was the change in daytime ambulatory systolic blood pressure (SBP) at 2 months post-procedure. The RADIANCE-HTN SOLO trial reported a significant reduction in SBP among those who underwent the renal denervation procedure, with a decrease of -8.5 mmHg, which was a between-group difference of -6.3 mmHg compared to the sham procedure, reaching statistical significance with a p-value of 0.0001.
Secondary outcomes included changes in daytime ambulatory diastolic blood pressure (DBP), which also saw a notable reduction. Patients in the denervation group experienced a decrease of -5.1 mmHg, translating to a between-group difference of -2.6 mmHg compared to the sham procedure, with a p-value of 0.01. The 24-hour ambulatory SBP followed suit, showing a reduction of -7.0 mmHg and a between-group difference of -4.1 mmHg, again significant with a p-value of 0.006.
Moreover, the trial examined the need for antihypertensive treatment after 2 months, finding that a significantly lower percentage of patients required antihypertensive therapy post-denervation (55%) compared to the sham group (79%), with a p-value of 0.002.
The RADIANCE-HTN SOLO trial’s conclusion was compelling: renal denervation using the Paradise endovascular ultrasound system resulted in a greater reduction in both SBP and DBP at 2 months when compared with a sham procedure. These results suggest that renal denervation could be a promising therapeutic option for patients with mild to moderate hypertension, potentially reducing the need for long-term medication use.
This trial’s outcomes have profound implications for the treatment paradigm of hypertension, especially in patients who are either intolerant to medications or wish to minimize medication use. It has sparked a new interest in renal denervation and its potential role in hypertension management. The RADIANCE-HTN SOLO trial underscores the potential of interventional procedures in controlling blood pressure and opens up avenues for further research in this domain.
The study’s robust design and clear outcomes support the notion that for a subset of hypertensive patients, renal denervation can offer significant clinical benefits. As hypertension remains a leading cause of cardiovascular morbidity and mortality worldwide, the findings of the RADIANCE-HTN SOLO trial contribute invaluable data towards evolving more effective and patient-tailored therapeutic strategies.