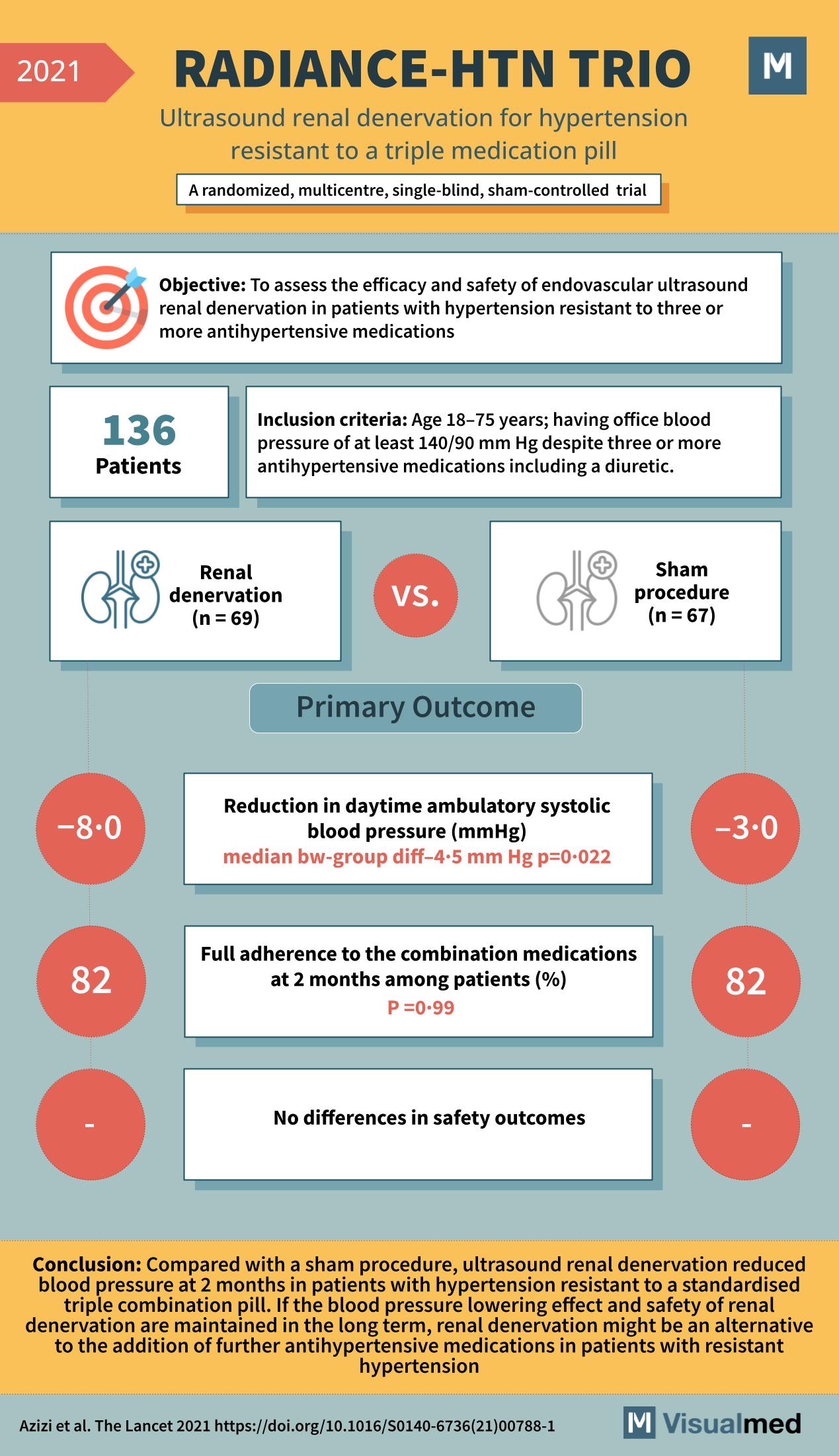
The RADIANCE-HTN TRIO trial, detailed in The Lancet in 2021, is another pivotal study in the domain of hypertension treatment, focusing on a challenging group of patients: those resistant to a standard triple combination pill. This trial delves into the assessment of endovascular ultrasound renal denervation as a treatment for resistant hypertension.
Conducted across multiple centers, the RADIANCE-HTN TRIO trial was a randomized, single-blind, sham-controlled trial including 136 patients. Participants ranged from 18 to 75 years old, all presenting with office blood pressure of at least 140/90 mm Hg despite being on a regimen of three or more antihypertensive medications, including a diuretic.
The primary objective was to evaluate the efficacy and safety of renal denervation in comparison to a sham procedure. The primary outcome measured was the reduction in daytime ambulatory systolic blood pressure (SBP) at 2 months post-intervention. The results were significant: the renal denervation group experienced an 8 mm Hg reduction in SBP, with a median between-group difference of 4.5 mm Hg (p=0.022), suggesting a marked improvement over the sham procedure.
In terms of medication adherence, there was no significant difference between the two groups at the 2-month mark, with both showing an 82% adherence rate to the combination medications, indicating that the observed reduction in SBP was not due to differences in medication intake.
Importantly, no differences in safety outcomes were noted, highlighting the non-inferiority of renal denervation compared to the sham procedure in terms of adverse events. This finding is crucial, considering the invasive nature of the procedure and the risks typically associated with such interventions.
The conclusion of the RADIANCE-HTN TRIO trial was profound. Ultrasound renal denervation led to a notable reduction in blood pressure at 2 months in patients with hypertension resistant to a standard triple medication pill. If the blood pressure-lowering effect and safety of renal denervation are maintained in the long term, this procedure could be considered an alternative to escalating pharmacological treatment in patients with resistant hypertension.
The implications of the RADIANCE-HTN TRIO trial are far-reaching. It offers hope for patients who have struggled to control their blood pressure despite using multiple medications. By demonstrating the effectiveness and safety of renal denervation, this trial supports the procedure as a viable therapeutic option, potentially altering the treatment landscape for resistant hypertension.
The trial also reinforces the importance of continued innovation in the field of cardiovascular medicine. The pursuit of alternative treatments, such as renal denervation, is essential for addressing the varying needs of hypertensive patients, particularly those who do not respond adequately to traditional pharmacotherapy.
The RADIANCE-HTN TRIO trial, therefore, not only advances our scientific understanding but also underscores the need for personalized treatment approaches. As hypertension remains a significant global health issue, the insights gained from this study will likely influence future guidelines and clinical practices in managing resistant hypertension.