Insights from the REDUCE LAP-HF II Trial: Atrial Shunt Devices in Heart Failure Management
The REDUCE LAP-HF II Trial, published in 2022, was a significant randomized, blinded, sham-controlled trial aiming to enhance the treatment for patients with heart failure. It specifically targeted those with preserved and mildly reduced ejection fraction to evaluate the impact of an atrial shunt device.
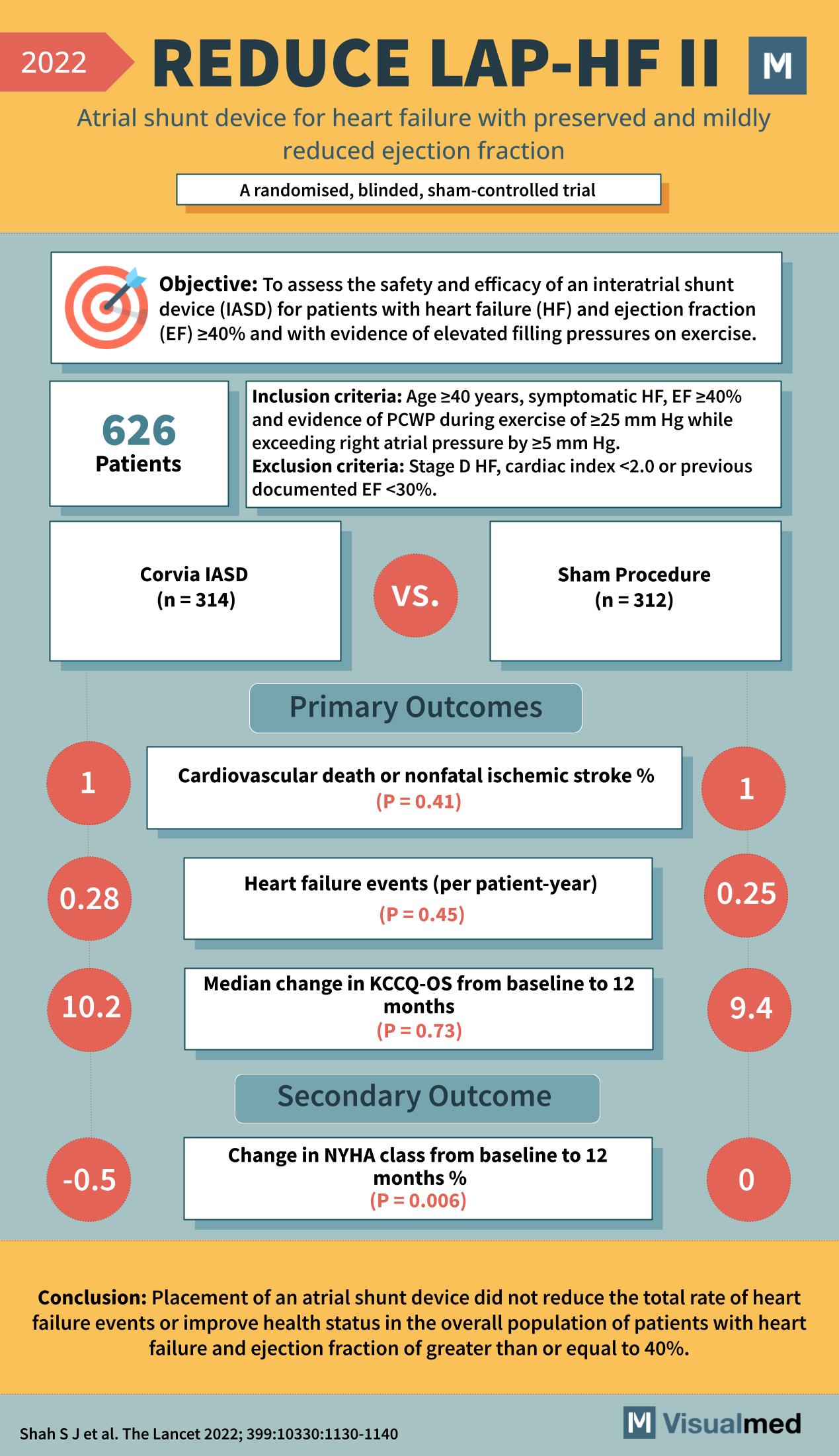
Objective of the REDUCE LAP-HF II Trial
The trial aimed to assess the safety and efficacy of an interatrial shunt device (IASD) for patients with heart failure (HF) who had an ejection fraction (EF) ≥40% and demonstrated elevated filling pressures during exercise.
Participant Criteria and Demographics
A total of 626 patients were enrolled in the study, with inclusion criteria requiring individuals aged ≥40 years, symptomatic heart failure, EF ≥40%, and evidence of pulmonary capillary wedge pressure (PCWP) of ≥25 mm Hg during exercise. Notably, those with Stage D heart failure, a cardiac index <2.0, or previously documented EF <30% were excluded.
The Approach of the REDUCE LAP-HF II Trial
Patients were randomized into two groups: 314 received the Corvia IASD, while 312 underwent a sham procedure.
Outcomes of the REDUCE LAP-HF II Trial
Primary Outcomes
The trial reported the following primary outcomes:
- The occurrence of cardiovascular death or nonfatal ischemic stroke was 1% in the IASD group compared to 1% in the sham procedure group (P = 0.41).
- Heart failure events per patient-year were slightly higher in the IASD group (0.28) compared to the sham group (0.25), but not statistically significant (P = 0.45).
- There was a median change in the Kansas City Cardiomyopathy Questionnaire-Overall Summary (KCCQ-OS) score from baseline to 12 months, with the IASD group experiencing a 10.2 point change versus a 9.4 point change in the sham group (P = 0.73).
Secondary Outcome
- There was a significant change in the New York Heart Association (NYHA) class from baseline to 12 months, with the IASD group showing a 0.5% improvement compared to no change in the sham group (P = 0.006).
Conclusion and Clinical Impact
The REDUCE LAP-HF II Trial concluded that the placement of an atrial shunt device did not reduce the total rate of heart failure events or improve health status in the overall population of patients with heart failure and EF ≥40%. The trial’s results contribute to the ongoing discussion about the role of atrial shunt devices in heart failure treatment, indicating that while some improvements in functional status can be seen, the primary metrics of heart failure management remain unchanged with this intervention.