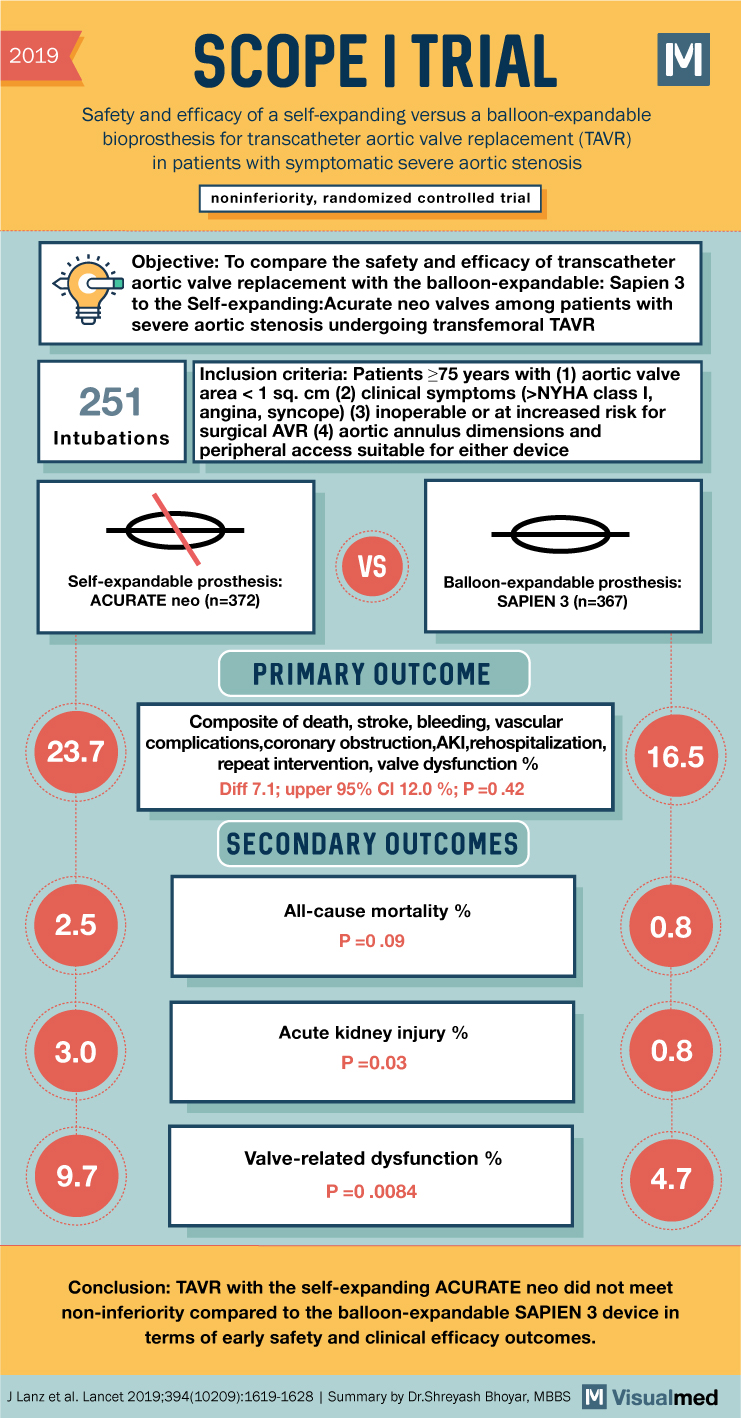
2019 SCOPE I TRIAL Safety and efficacy of a self-expanding versus a balloon-expandable bioprosthesis for transcatheter aortic valve replacement (TAVR) in patients with symptomatic severe aortic stenosis noninferiority, randomized controlled trial M Objective: To compare the safety and efficacy of transcatheter aortic valve replacement with the balloon-expandable: Sapien 3 to the Self-expanding:Acurate neo valves among patients with severe aortic stenosis undergoing transfemoral TAVR 251 Intubations Inclusion criteria: Patients ≥75 years with (1) aortic valve area < 1 sq. cm (2) clinical symptoms (>NYHA class I, angina, syncope) (3) inoperable or at increased risk for surgical AVR (4) aortic annulus dimensions and peripheral access suitable for either device VS Self-expandable prosthesis: ACURATE neo (n=372) Balloon-expandable prosthesis: SAPIEN 3 (n=367) 23.7 PRIMARY OUTCOME Composite of death, stroke, bleeding, vascular complications,coronary obstruction,AKI,rehospitalization, repeat intervention, valve dysfunction % Diff 7.1; upper 95% CI 12.0%; P=0.42 16.5 SECONDARY OUTCOMES All-cause mortality % 2.5 0.8 P=0.09 3.0 Acute kidney injury % P=0.03 0.8 Valve-related dysfunction % 9.7 4.7 P=0.0084 Conclusion: TAVR with the self-expanding ACURATE neo did not meet non-inferiority compared to the balloon-expandable SAPIEN 3 device in terms of early safety and clinical efficacy outcomes. J Lanz et al. Lancet 2019;394(10209):1619-1628