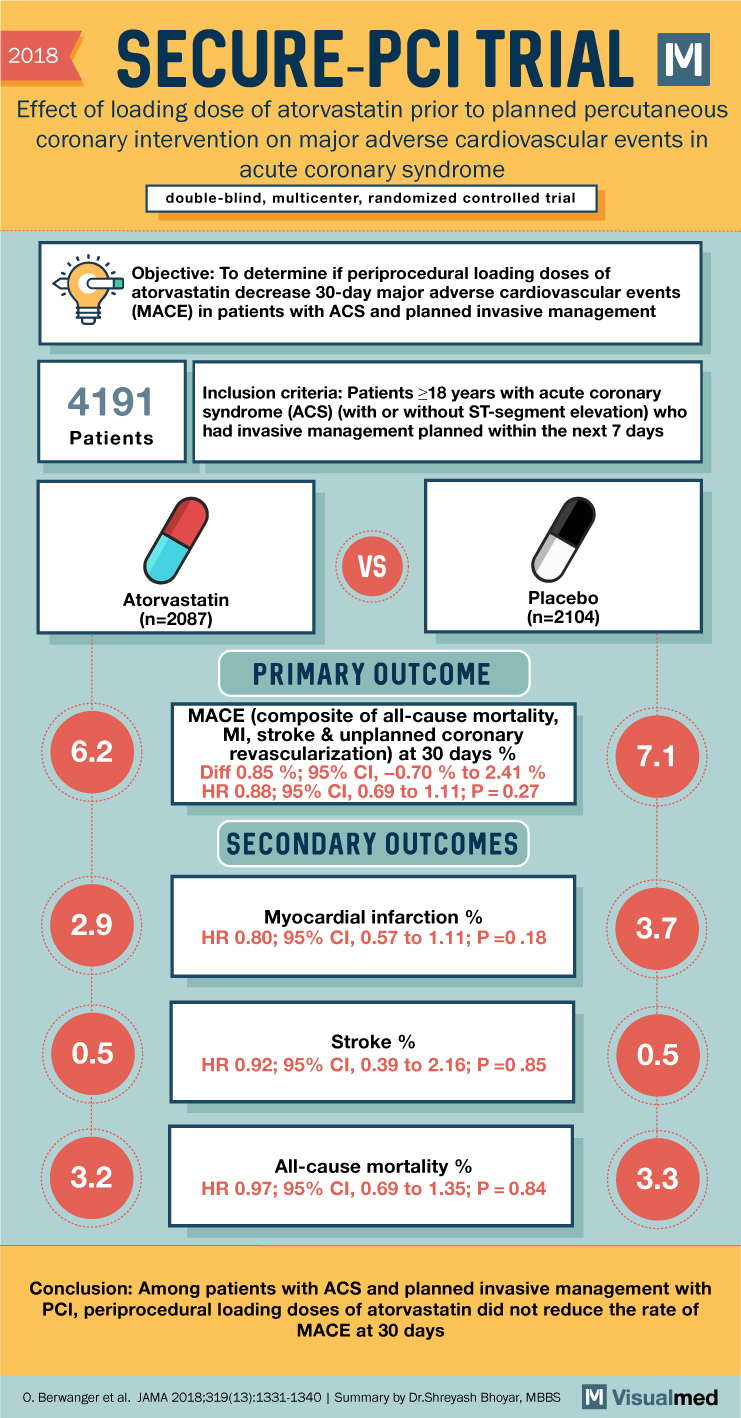
2018 SECURE-PCI TRIAL Effect of loading dose of atorvastatin prior to planned percutaneous coronary intervention on major adverse cardiovascular events in acute coronary syndrome double-blind, multicenter, randomized controlled trial Objective: To determine if periprocedural loading doses of atorvastatin decrease 30-day major adverse cardiovascular events (MACE) in patients with ACS and planned invasive management 4191 Patients Inclusion criteria: Patients ≥18 years with acute coronary syndrome (ACS) (with or without ST-segment elevation) who had invasive management planned within the next 7 days VS Atorvastatin (n=2087) PRIMARY OUTCOME Placebo (n=2104) MACE (composite of all-cause mortality, MI, stroke & unplanned coronary revascularization) at 30 days % 6.2 7.1 Diff 0.85 %; 95% CI, -0.70 % to 2.41 % HR 0.88; 95% CI, 0.69 to 1.11; P=0.27 SECONDARY OUTCOMES Myocardial infarction % 2.9 3.7 HR 0.80; 95% CI, 0.57 to 1.11; P=0.18 Stroke % 0.5 0.5 HR 0.92; 95% CI, 0.39 to 2.16; P=0.85 All-cause mortality % 3.2 3.3 HR 0.97; 95% CI, 0.69 to 1.35; P = 0.84 Conclusion: Among patients with ACS and planned invasive management with PCI, periprocedural loading doses of atorvastatin did not reduce the rate of MACE at 30 days O. Berwanger et al. JAMA 2018;319(13):1331-1340