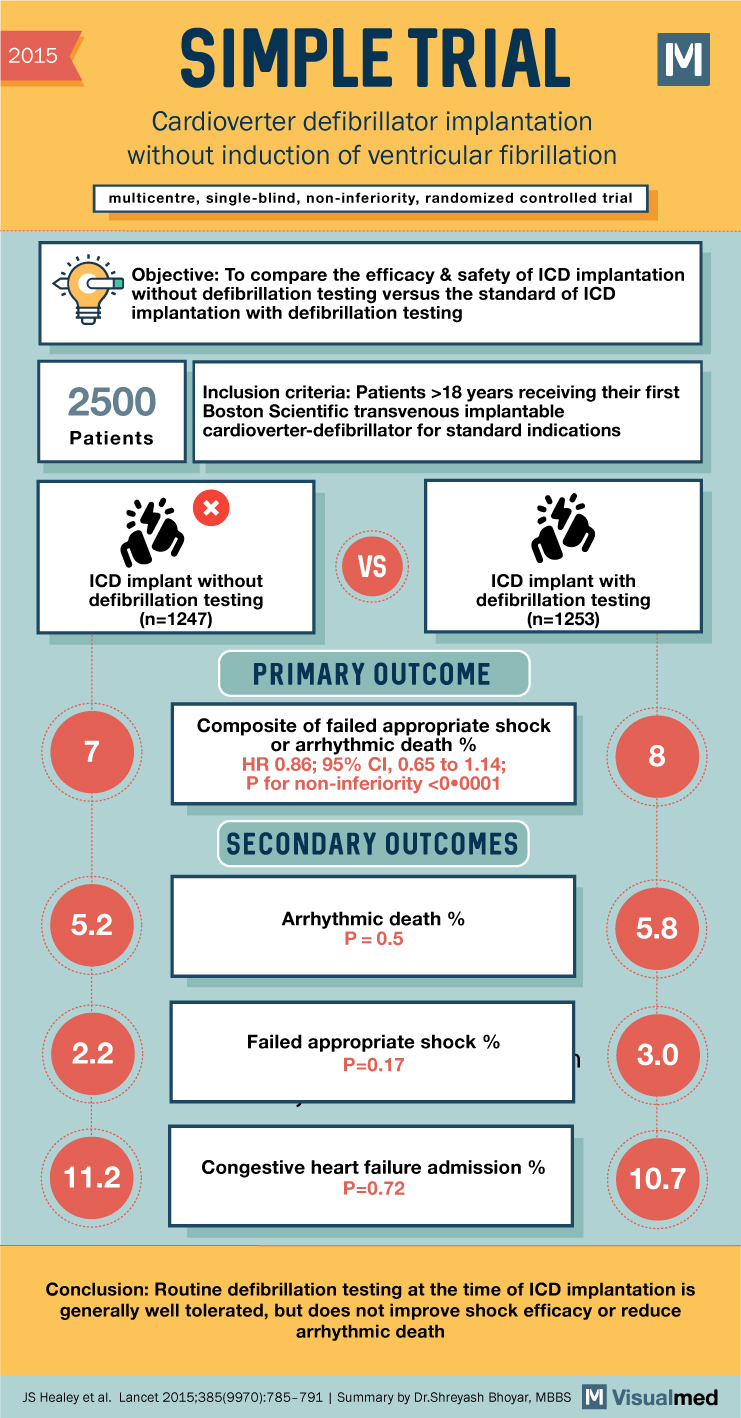
2015 SIMPLE TRIAL Cardioverter defibrillator implantation without induction of ventricular fibrillation multicentre, single-blind, non-inferiority, randomized controlled trial M Objective: To compare the efficacy & safety of ICD implantation without defibrillation testing versus the standard of ICD implantation with defibrillation testing 2500 Patients Inclusion criteria: Patients >18 years receiving their first Boston Scientific transvenous implantable cardioverter-defibrillator for standard indications x VS ICD implant without defibrillation testing (n=1247) 7 ICD implant with defibrillation testing (n=1253) PRIMARY OUTCOME Composite of failed appropriate shock or arrhythmic death % HR 0.86; 95% CI, 0.65 to 1.14; P for non-inferiority <0.0001 SECONDARY OUTCOMES 5.2 8 Arrhythmic death % 5.8 P=0.5 2.2 Failed appropriate shock % P=0.17 3.0 11.2 Congestive heart failure admission % 10.7 P=0.72 Conclusion: Routine defibrillation testing at the time of ICD implantation is generally well tolerated, but does not improve shock efficacy or reduce arrhythmic death JS Healey et al. Lancet 2015;385(9970):785-791