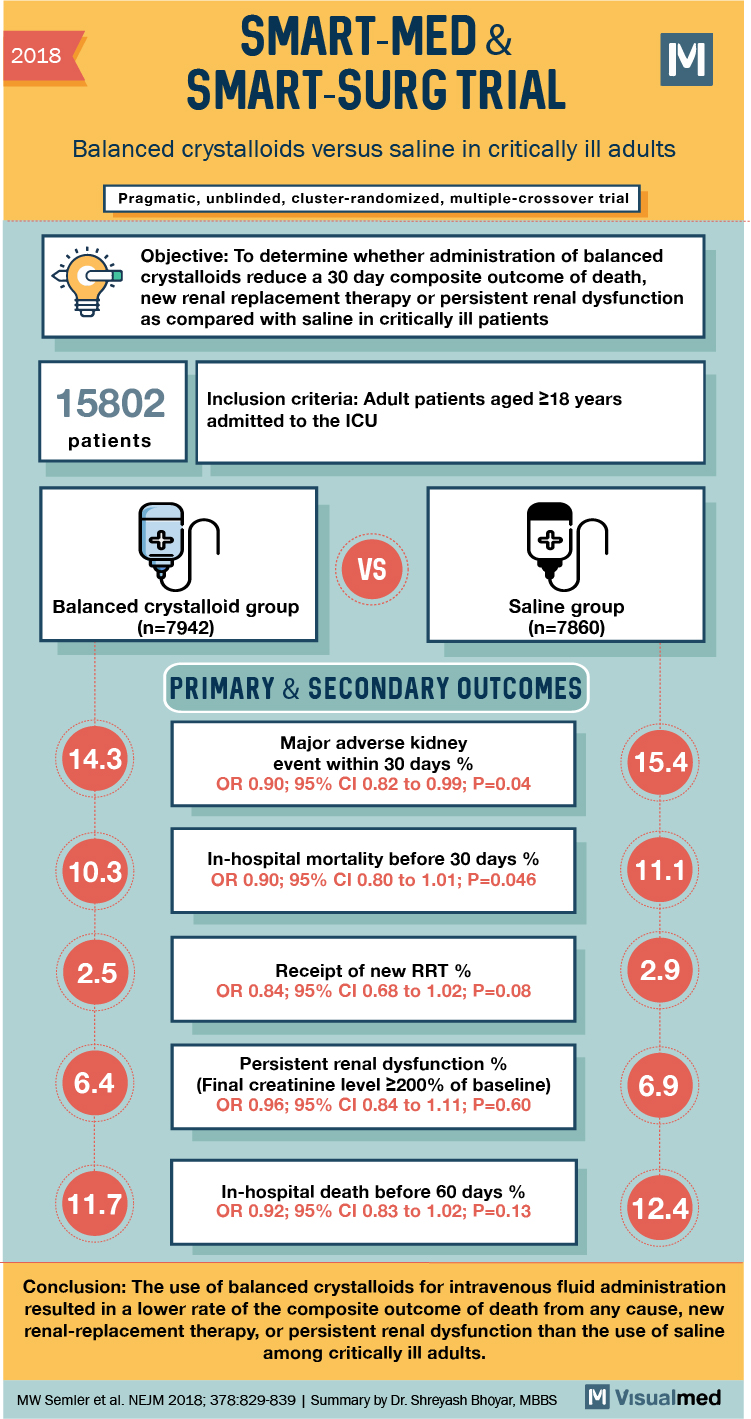
2018 SMART-MED & SMART-SURG TRIAL Balanced crystalloids versus saline in critically ill adults Pragmatic, unblinded, cluster-randomized, multiple-crossover trial Objective: To determine whether administration of balanced crystalloids reduce a 30 day composite outcome of death, new renal replacement therapy or persistent renal dysfunction as compared with saline in critically ill patients 15802 patients Inclusion criteria: Adult patients aged 218 years admitted to the ICU Balanced crystalloid group (n=7942) Saline group (n=7860) PRIMARY & SECONDARY OUTCOMES 14.3 Major adverse kidney event within 30 days % OR 0.90; 95% CI 0.82 to 0.99; P=0.04 15.4 10.3 In-hospital mortality before 30 days % OR 0.90; 95% CI 0.80 to 1.01; P=0.046 11.1 2.5 Receipt of new RRT % OR 0.84; 95% CI 0.68 to 1.02; P=0.08 2.9 6.4 Persistent renal dysfunction % (Final creatinine level 2200% of baseline) OR 0.96; 95% CI 0.84 to 1.11; P=0.60 6.9 11.7 In-hospital death before 60 days % OR 0.92; 95% CI 0.83 to 1.02; P=0.13 Conclusion: The use of balanced crystalloids for intravenous fluid administration resulted in a lower rate of the composite outcome of death from any cause, new renal-replacement therapy, or persistent renal dysfunction than the use of saline among critically ill adults. MW Semler et al. NEJM 2018; 378:829-839