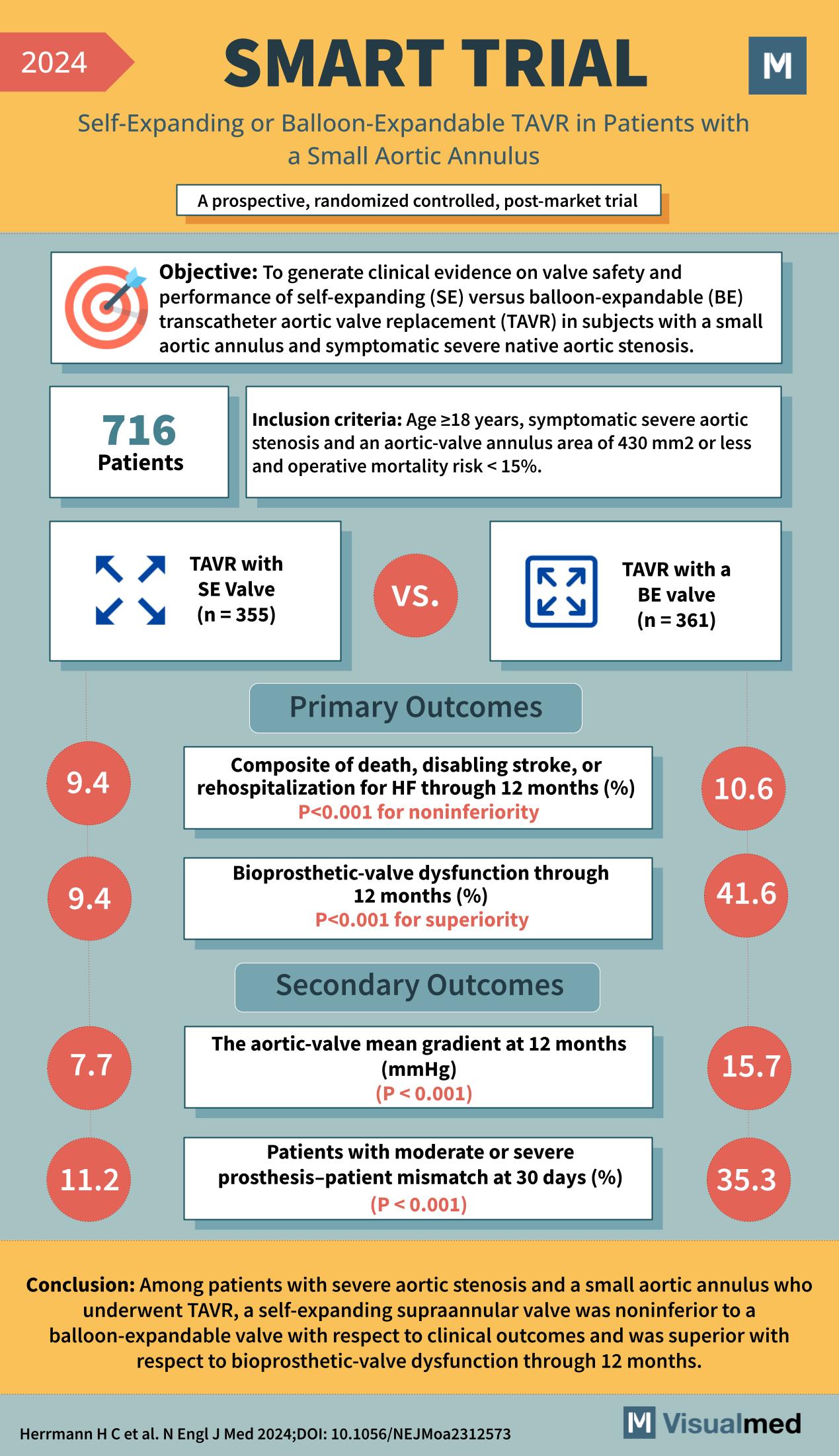
Year: 2024 Title: SMART TRIAL Subtitle: Self-Expanding or Balloon-Expandable TAVR in Patients with a Small Aortic Annulus Type of Trial: A prospective, randomized controlled, post-market trial
Objective: To generate clinical evidence on valve safety and performance of self-expanding (SE) versus balloon-expandable (BE) transcatheter aortic valve replacement (TAVR) in subjects with a small aortic annulus and symptomatic severe native aortic stenosis.
Patients: 716
Inclusion Criteria:
- Age ≥18 years
- Symptomatic severe aortic stenosis
- Aortic-valve annulus area of 430 mm2 or less
- Operative mortality risk <15%
Groups:
- TAVR with SE Valve (n = 355)
- TAVR with a BE valve (n = 361)
Primary Outcomes:
- Composite of death, disabling stroke, or rehospitalization for HF through 12 months (%)
- SE Valve: 9.4%
- BE Valve: 10.6%
- P<0.001 for noninferiority
- Bioprosthetic-valve dysfunction through 12 months (%)
- SE Valve: 9.4%
- BE Valve: 41.6%
- P<0.001 for superiority
Secondary Outcomes:
- The aortic-valve mean gradient at 12 months (mmHg)
- SE Valve: 7.7
- BE Valve: 15.7
- P < 0.001
- Patients with moderate or severe prosthesis–patient mismatch at 30 days (%)
- SE Valve: 11.2%
- BE Valve: 35.3%
- P < 0.001
Conclusion: Among patients with severe aortic stenosis and a small aortic annulus who underwent TAVR, a self-expanding supraannular valve was noninferior to a balloon-expandable valve with respect to clinical outcomes and was superior with respect to bioprosthetic-valve dysfunction through 12 months.
Reference: Herrmann HC et al. N Engl J Med 2024; DOI: 10.1056/NEJMoa2312573