TAME Trial Summary
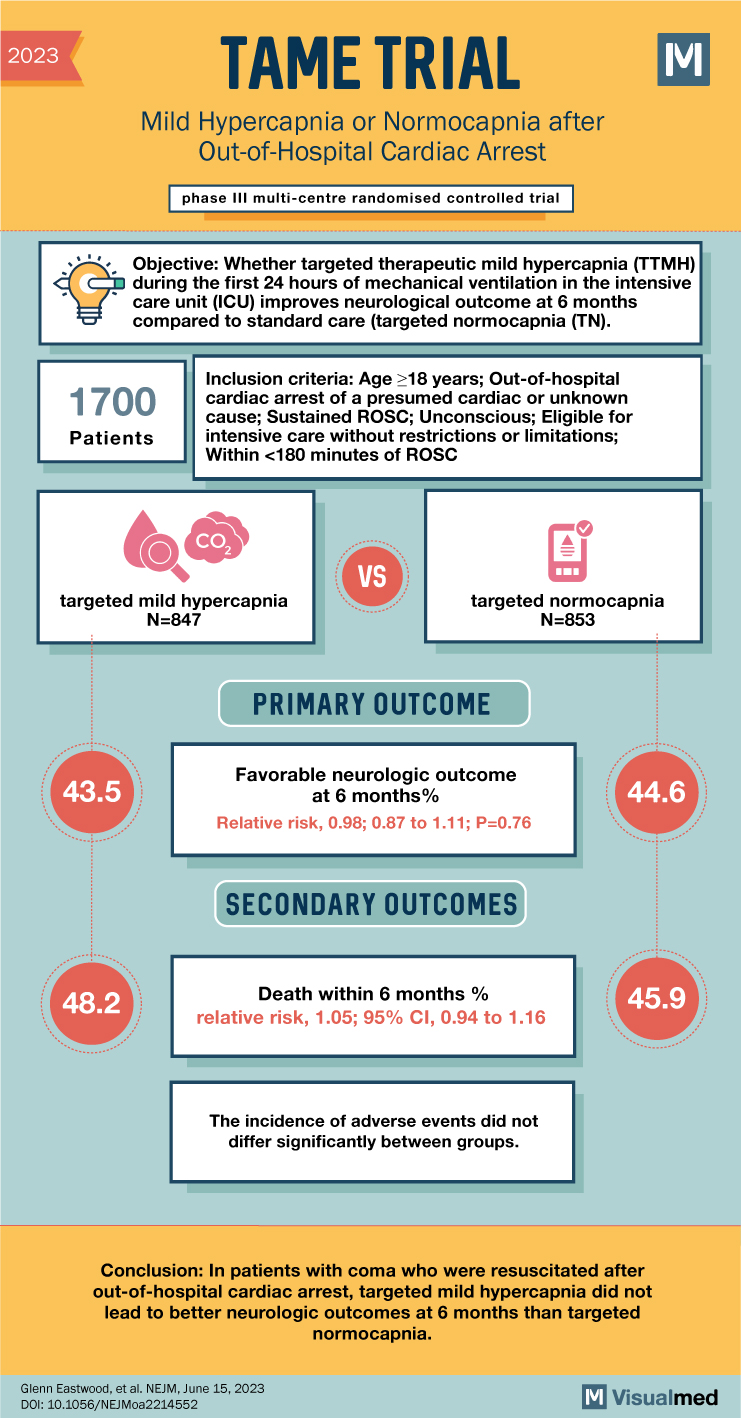
The TAME trial investigated the impact of mild hypercapnia versus normocapnia on neurologic outcomes in adults with coma who were resuscitated after out-of-hospital cardiac arrest. Current guidelines recommend normocapnia, but mild hypercapnia has been suggested to enhance cerebral blood flow and potentially improve neurologic outcomes.
The trial randomly assigned adults admitted to the intensive care unit (ICU) after out-of-hospital cardiac arrest to either 24 hours of mild hypercapnia (target partial pressure of arterial carbon dioxide [Paco2] between 50 and 55 mm Hg) or normocapnia (target Paco2 between 35 and 45 mm Hg) in a 1:1 ratio. The primary outcome assessed was a favorable neurologic outcome at 6 months, defined as a score of 5 or higher on the Glasgow Outcome Scale–Extended, indicating lower moderate disability or better. The secondary outcome measured was death within 6 months.
The trial included a total of 1,700 patients from 63 ICUs in 17 countries, with 847 patients assigned to the mild hypercapnia group and 853 to the normocapnia group. At 6 months, a favorable neurologic outcome occurred in 43.5% of patients in the mild hypercapnia group and 44.6% in the normocapnia group. The relative risk of achieving a favorable neurologic outcome with mild hypercapnia compared to normocapnia was 0.98, with a 95% confidence interval (CI) of 0.87 to 1.11. This result indicated no significant difference between the two groups (P=0.76). Regarding death within 6 months after randomization, it occurred in 48.2% of patients in the mild hypercapnia group and 45.9% in the normocapnia group, with a relative risk of 1.05 (95% CI, 0.94 to 1.16). The incidence of adverse events did not significantly differ between the groups.
In conclusion, the TAME trial found that among patients with coma who were resuscitated after out-of-hospital cardiac arrest, targeted mild hypercapnia did not result in better neurologic outcomes at 6 months compared to targeted normocapnia. There was no significant difference between the two groups in terms of achieving a favorable neurologic outcome or death within 6 months. Furthermore, the incidence of adverse events did not differ significantly between the mild hypercapnia and normocapnia groups. These findings indicate that mild hypercapnia does not confer an advantage over normocapnia in improving neurologic outcomes in this specific patient population. These results have important implications for clinical practice and highlight the need to adhere to current guidelines recommending normocapnia in adults with coma following out-of-hospital cardiac arrest.