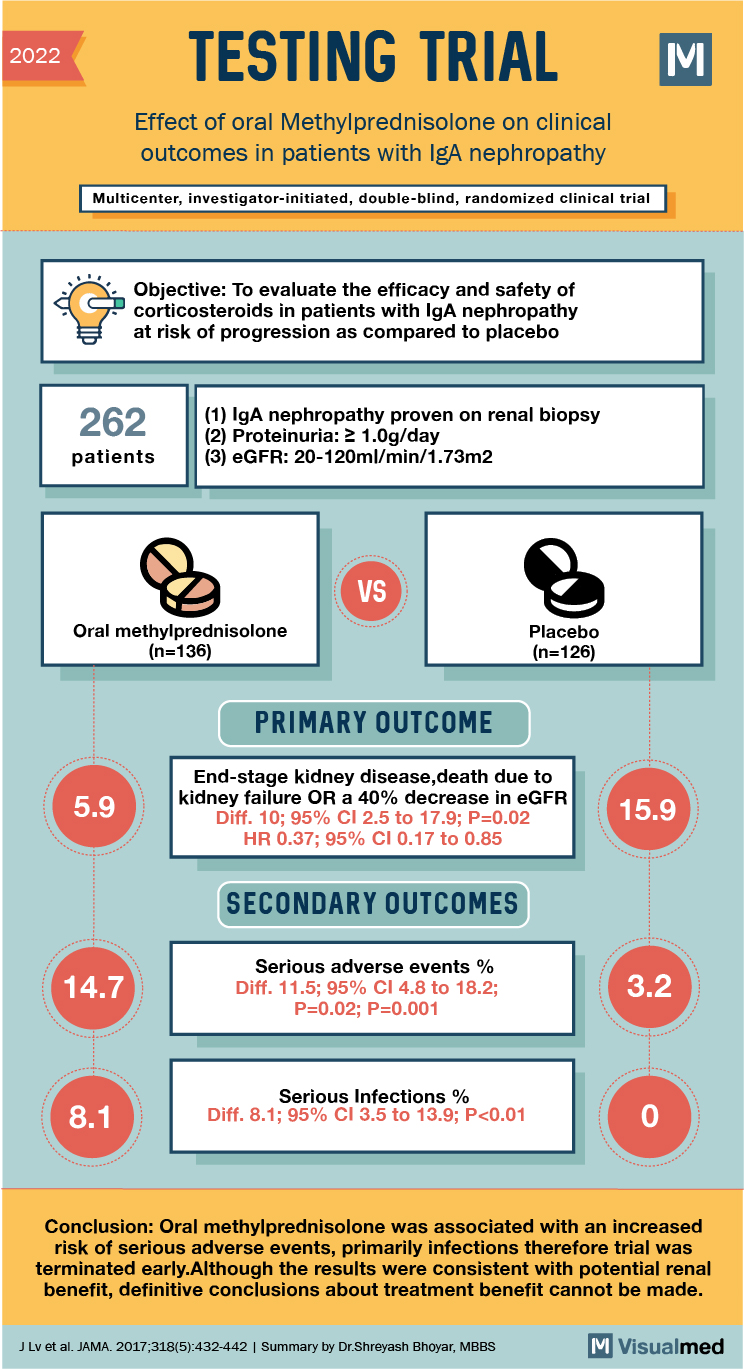
2022 TESTING TRIAL Effect of oral Methylprednisolone on clinical outcomes in patients with IgA nephropathy Multicenter, investigator-initiated, double-blind, randomized clinical trial Objective: To evaluate the efficacy and safety of corticosteroids in patients with IgA nephropathy at risk of progression as compared to placebo 262 (1) IgA nephropathy proven on renal biopsy (2) Proteinuria:2 1.0g/day (3) eGFR: 20-120ml/min/1.73m2 patients VS Oral methylprednisolone (n=136) Placebo (n=126) PRIMARY OUTCOME 5.9 End-stage kidney disease,death due to kidney failure OR a 40% decrease in eGFR Diff. 10; 95% CI 2.5 to 17.9; P=0.02 HR 0.37; 95% CI 0.17 to 0.85 15.9 SECONDARY OUTCOMES 14.7 Serious adverse events % Diff. 11.5; 95% CI 4.8 to 18.2; P=0.02; P=0.001 3.2 Serious Infections % Diff. 8.1; 95% CI 3.5 to 13.9; P<0.01 Conclusion: Oral methylprednisolone was associated with an increased risk of serious adverse events, primarily infections therefore trial was terminated early.Although the results were consistent with potential renal benefit, definitive conclusions about treatment benefit cannot be made. J Lv et al. JAMA. 2017;318(5):432-442