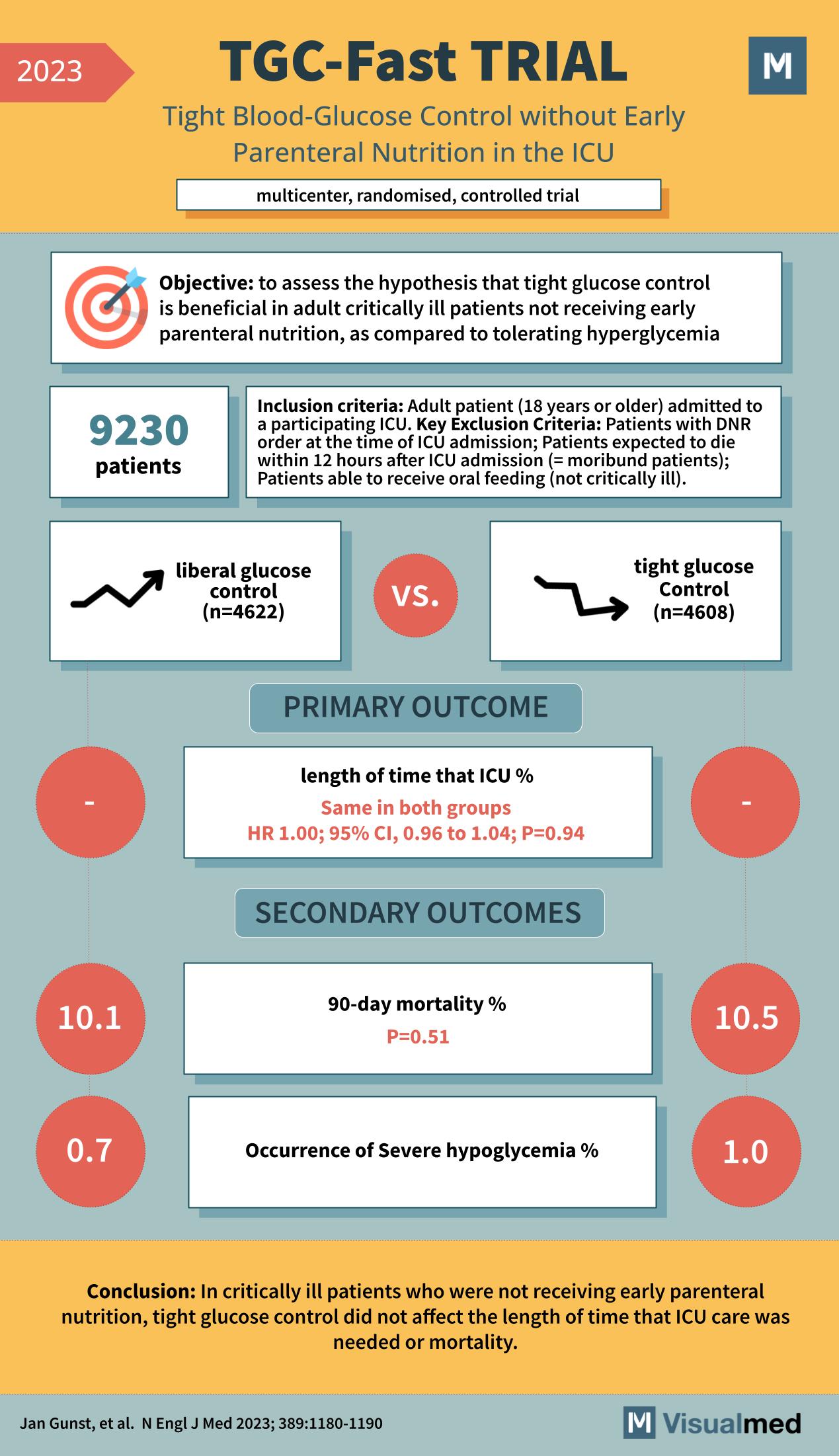
The TGC-Fast Trial, featured in the New England Journal of Medicine in 2023, was a multicenter, randomized, controlled trial that aimed to assess the benefits of tight glucose control without early parenteral nutrition in the intensive care unit (ICU). The study included 9,230 adult critically ill patients who were not receiving early parenteral nutrition and compared the effects of tight glucose control to a liberal glucose control strategy.
The study divided the patients into two groups: 4,622 patients received liberal glucose control, and 4,608 received tight glucose control. The primary outcome was the length of time that ICU care was needed, and it was found to be the same in both groups with a hazard ratio (HR) of 1.00 (95% CI, 0.96 to 1.04; P=0.94), indicating no significant difference between the two strategies.
Secondary outcomes included 90-day mortality and the occurrence of severe hypoglycemia. The 90-day mortality rates were similar between the two groups, with 10.1% in the liberal glucose control group and 10.5% in the tight control group (P=0.51). Severe hypoglycemia was less common in the liberal control group (0.7%) compared to the tight control group (1.0%).
The conclusion of the TGC-Fast Trial was that in critically ill patients not receiving early parenteral nutrition, tight glucose control did not affect the length of time that ICU care was required or mortality. These findings suggest that for critically ill patients, tight glucose control may not provide significant benefits over a more liberal approach to glucose management in the ICU setting. The results of this trial provide valuable information for clinicians managing glucose levels in critically ill patients, particularly regarding the risks and benefits of more stringent glucose control measures.