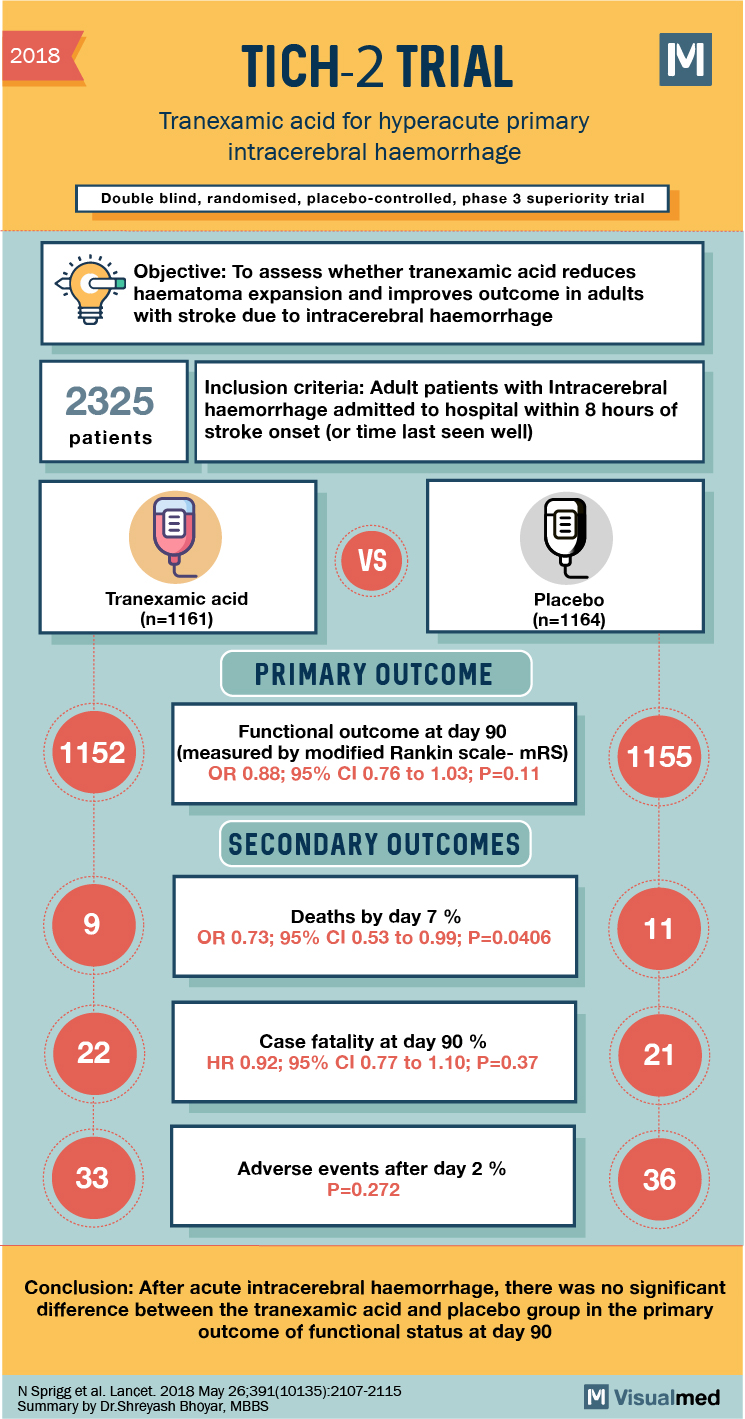
2018 TICH-2 TRIAL Tranexamic acid for hyperacute primary intracerebral haemorrhage Double blind, randomised, placebo-controlled, phase 3 superiority trial Objective: To assess whether tranexamic acid reduces haematoma expansion and improves outcome in adults with stroke due to intracerebral haemorrhage 3 2325 Inclusion criteria: Adult patients with Intracerebral haemorrhage admitted to hospital within 8 hours of stroke onset (or time last seen well) patients Tranexamic acid (n=1161) Placebo (n=1164) PRIMARY OUTCOME 1152 Functional outcome at day 90 (measured by modified Rankin scale-mRS) OR 0.88; 95% CI 0.76 to 1.03; P=0.11 1155 SECONDARY OUTCOMES Deaths by day 7 % OR 0.73; 95% CI 0.53 to 0.99; P=0.0406 Case fatality at day 90 % HR 0.92; 95% CI 0.77 to 1.10; P=0.37 Adverse events after day 2 % P=0.272 Conclusion: After acute intracerebral haemorrhage, there was no significant difference between the tranexamic acid and placebo group in the primary outcome of functional status at day 90 N Sprigg et al. Lancet 2018 May 26,391(10135) 2107-2115