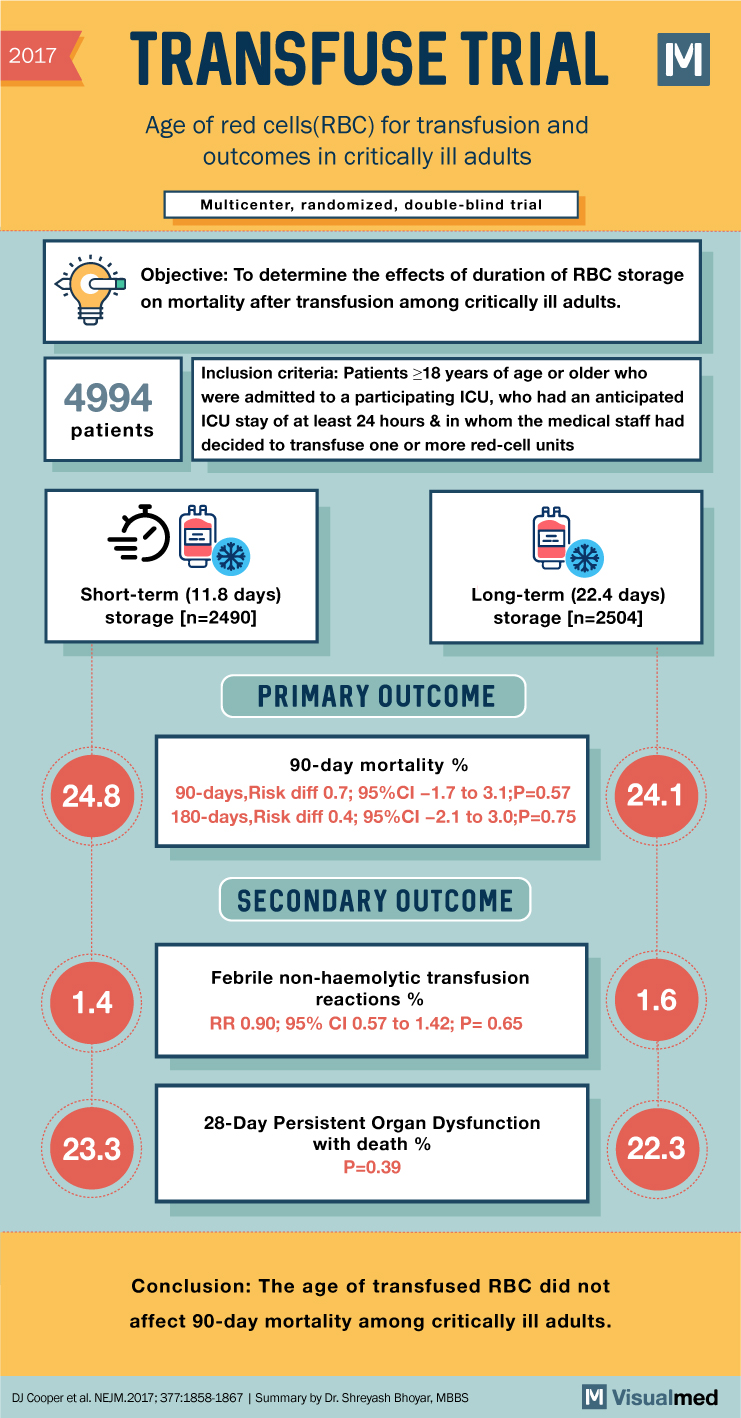
2017 TRANSFUSE TRIAL Age of red cells(RBC) for transfusion and outcomes in critically ill adults Multicenter, randomized, double-blind trial Objective: To determine the effects of duration of RBC storage on mortality after transfusion among critically ill adults. 4994 patients Inclusion criteria: Patients >18 years of age or older who were admitted to a participating ICU, who had an anticipated ICU stay of at least 24 hours & in whom the medical staff had decided to transfuse one or more red-cell units Short-term (11.8 days) storage (n=2490] Long-term (22.4 days) storage (n=2504] PRIMARY OUTCOME 24.8 90-day mortality % 90-days, Risk diff 0.7; 95%CI -1.7 to 3.1;P=0.57 180-days, Risk diff 0.4; 95%CI -2.1 to 3.0;P=0.75 24.1 SECONDARY OUTCOME 1.4 Febrile non-haemolytic transfusion reactions % RR 0.90; 95% CI 0.57 to 1.42; P= 0.65 1.6 23.3 28-Day Persistent Organ Dysfunction with death % P=0.39 Conclusion: The age of transfused RBC did not affect 90-day mortality among critically ill adults. DJ Cooper et al. NEJM.2017:377:1858-1867 | Summary by Dr. Shreyash Bhoyar, MBBS