VITAL-AF Trial Summary
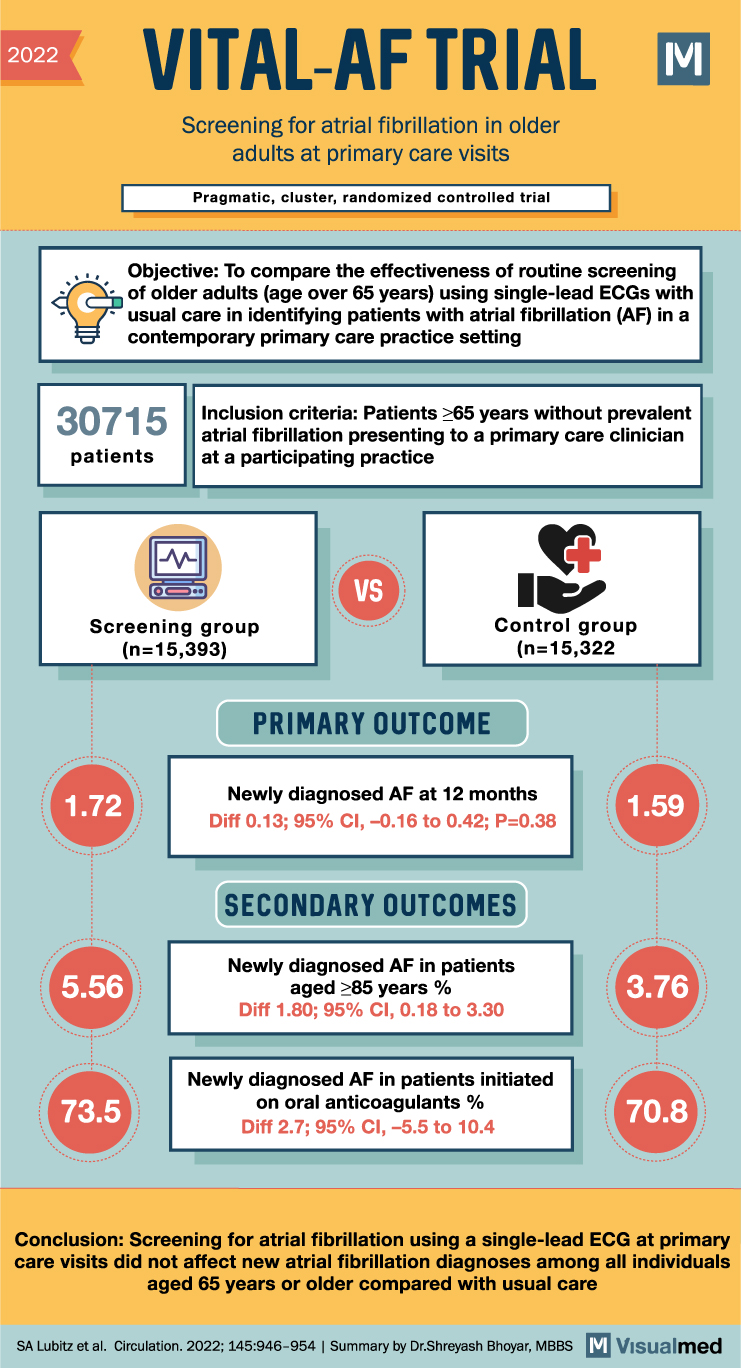
The VITAL-AF trial aimed to determine whether point-of-care screening with a handheld single-lead ECG device during primary care visits increases the diagnosis of atrial fibrillation (AF) in older adults. The study randomized 16 primary care clinics in a 1:1 ratio, with half implementing AF screening using the AliveCor KardiaMobile device during vital sign assessments, while the other half provided usual care without systematic screening. The participants included were aged 65 years or older, and screening results were communicated to the primary care clinicians during the patient encounters. Diagnostic testing and treatment decisions were made by the clinicians, and new AF diagnoses during the 1-year follow-up were ascertained and adjudicated.
Among the 30,715 patients without prevalent AF at baseline (15,393 in the screening group and 15,322 in the control group), the incidence of new AF diagnoses at 1 year was 1.72% in the screening group and 1.59% in the control group, indicating no significant difference between the two groups (risk difference, 0.13%; 95% confidence interval [CI], -0.16 to 0.42; P=0.38). However, subgroup analyses revealed that in individuals aged 85 years or older, the rate of new AF diagnoses was higher in both the screening and control groups compared to the overall population (5.56% versus 3.76%, respectively; risk difference, 1.80%; 95% CI, 0.18 to 3.30).
When comparing the screening period to the previous year, the difference in newly diagnosed AF between the screening and control groups was slightly larger in the screening group (0.32% versus -0.12%; risk difference, 0.43%; 95% CI, -0.01 to 0.84). However, this difference was not statistically significant. The proportion of individuals with newly diagnosed AF who were initiated on oral anticoagulants was similar in the screening (73.5%) and control (70.8%) arms (risk difference, 2.7%; 95% CI, -5.5 to 10.4).
In conclusion, the VITAL-AF trial did not find evidence to support the use of point-of-care AF screening with a handheld single-lead ECG device during primary care visits to increase the diagnosis of new AF among individuals aged 65 years or older compared to usual care. Although subgroup analyses indicated higher rates of new AF diagnoses in older individuals, overall, the screening intervention did not significantly impact the detection of AF or the initiation of oral anticoagulant treatment. Further research may be necessary to explore the effectiveness of alternative screening strategies or targeted approaches for specific age groups.