WHiTE 5 Trial Summary
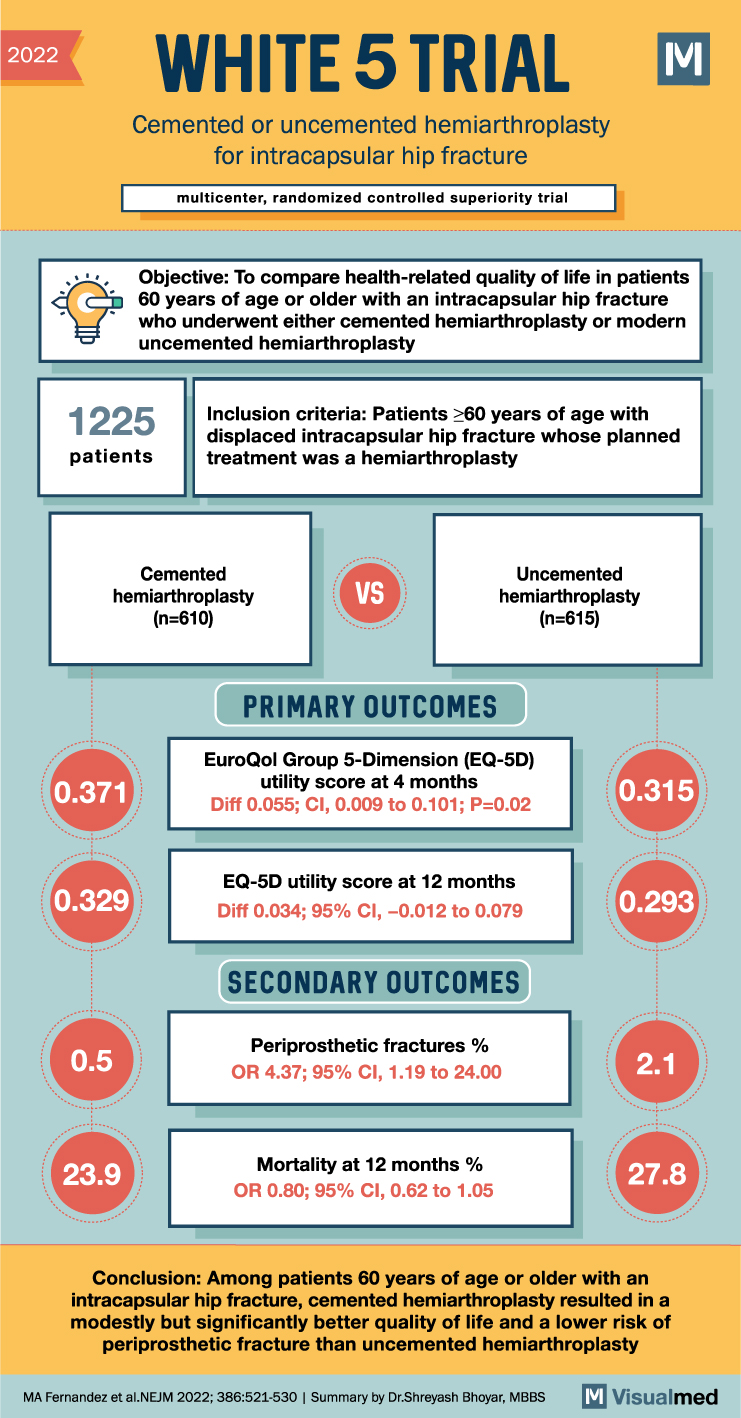
The WHiTE5 trial, a multicenter, randomized, controlled trial, has provided valuable insights into the use of bone cement in hip fractures treated with hemiarthroplasty. The trial aimed to compare the health-related quality of life and outcomes of patients undergoing cemented versus uncemented hemiarthroplasty for intracapsular hip fractures in individuals aged 60 years or older.
Traditionally, the use of bone cement in hemiarthroplasty has been a topic of debate, with limited data available on the long-term quality of life outcomes. In this study, 610 patients were assigned to undergo cemented hemiarthroplasty, while 615 patients were assigned to undergo modern uncemented hemiarthroplasty. Follow-up data at 4 months were available for 71.6% of the patients.
The primary outcome measure was health-related quality of life, assessed using utility scores from the EuroQol Group 5-Dimension (EQ-5D) questionnaire at 4 months after randomization. The EQ-5D utility score ranges from -0.594 to 1, with higher scores indicating better quality of life. The minimal clinically important difference ranges from 0.050 to 0.075.
The results of the trial demonstrated a significant difference in health-related quality of life between the two groups. The mean EQ-5D utility score was 0.371 in the cemented group and 0.315 in the uncemented group, with an adjusted difference of 0.055 (95% confidence interval [CI], 0.009 to 0.101; P=0.02). This indicates that patients undergoing cemented hemiarthroplasty had a modestly better quality of life compared to those undergoing uncemented hemiarthroplasty.
The between-group difference observed at 1 month was consistent with the 4-month results. However, the difference in quality of life between the two groups at 12 months was smaller than at 4 months. Additionally, the trial evaluated mortality rates at 12 months, which were 23.9% in the cemented group and 27.8% in the uncemented group. The odds ratio for death was 0.80 (95% CI, 0.62 to 1.05), indicating a slightly lower mortality risk in the cemented group, although the difference was not statistically significant.
Furthermore, the trial assessed the occurrence of periprosthetic fractures, a potential complication of hemiarthroplasty. The incidence of periprosthetic fractures was 0.5% in the cemented group and 2.1% in the uncemented group, with an odds ratio of 4.37 (95% CI, 1.19 to 24.00), indicating a significantly lower risk of periprosthetic fractures in the cemented group.
In conclusion, the WHiTE5 trial has provided important evidence regarding the use of bone cement in hemiarthroplasty for intracapsular hip fractures. Among patients aged 60 years or older, cemented hemiarthroplasty was associated with a modest but significant improvement in health-related quality of life compared to uncemented hemiarthroplasty. Additionally, the use of cemented hemiarthroplasty was associated with a lower risk of periprosthetic fractures. These findings have implications for the selection of surgical techniques and the optimization of outcomes in patients undergoing hemiarthroplasty for intracapsular hip fractures.