WOEST Trial Summary
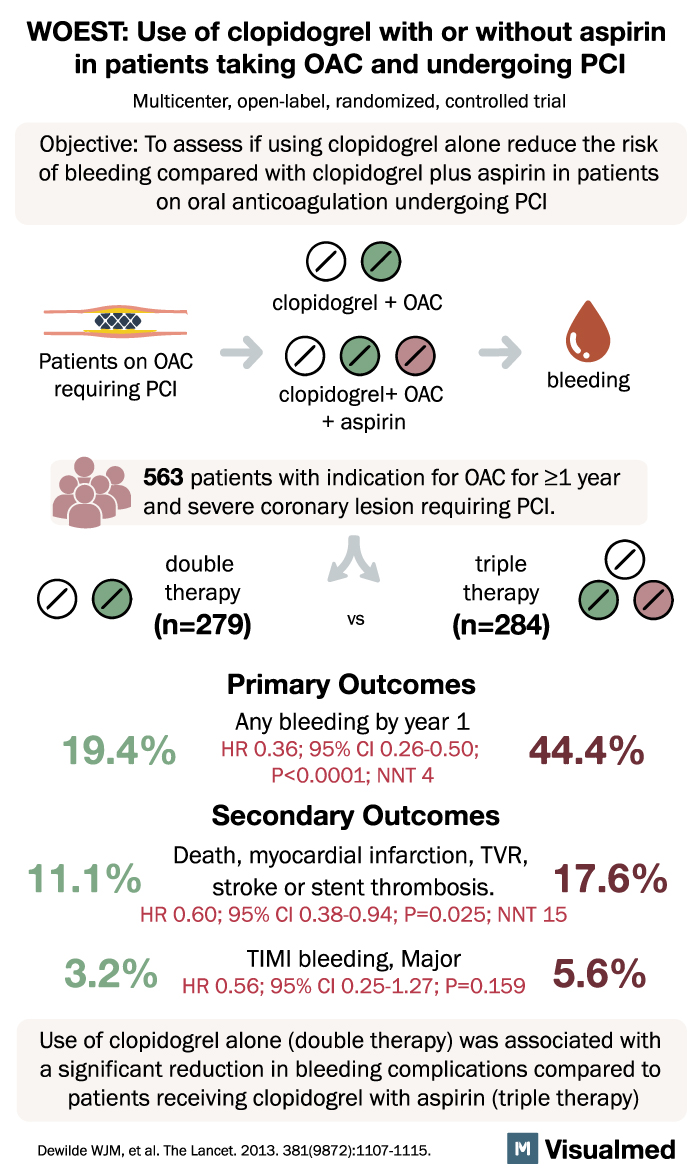
The WOEST trial, a groundbreaking study published in The Lancet, has shed light on a significant issue faced by patients undergoing percutaneous coronary intervention (PCI) while taking oral anticoagulants. Traditionally, triple therapy involving aspirin and clopidogrel has been recommended, but it comes with an increased risk of severe bleeding. The trial aimed to compare the safety and efficacy of clopidogrel alone (double therapy) versus clopidogrel plus aspirin (triple therapy).
Conducted from November 2008 to November 2011, this open-label, multicenter, randomized, controlled trial enrolled 573 adult patients receiving oral anticoagulants and undergoing PCI across 15 centers in Belgium and the Netherlands. The primary outcome assessed was any bleeding episode within one year of PCI, using an intention-to-treat approach. Out of the total patients, 279 (98.2%) were assigned double therapy, and 284 (98.3%) were assigned triple therapy.
The results were striking. In the double-therapy group, only 19.4% of patients experienced bleeding episodes, compared to 44.4% in the triple-therapy group. This marked reduction in bleeding complications was highly significant (hazard ratio [HR] 0.36, 95% CI 0.26-0.50, p<0.0001). Furthermore, the double-therapy group showed fewer instances of multiple bleeding events (2.2% vs. 12.0%) and a lower need for blood transfusions (3.9% vs. 9.5%) compared to the triple-therapy group (odds ratio from Kaplan-Meier curve 0.39, 95% CI 0.17-0.84, p=0.011).
The trial’s findings provide valuable insights. The use of clopidogrel without aspirin was associated with a significant reduction in bleeding complications, with no observed increase in thrombotic events. This outcome suggests that clopidogrel alone is a safer alternative for patients on oral anticoagulants undergoing PCI, as it effectively minimizes the risk of bleeding without compromising thrombotic protection.
The authors of the study highlight the clear advantage of clopidogrel monotherapy over triple therapy. They emphasize that administering clopidogrel alone to patients taking oral anticoagulants requiring PCI results in a significantly lower rate of bleeding complications at the one-year mark compared to the use of clopidogrel plus aspirin. Additionally, the trial did not identify any evidence of an increased risk of thrombotic events associated with withholding aspirin.
Although the trial’s sample size was relatively small, the implications of its findings are noteworthy. The results challenge the conventional approach of triple therapy and provide clinicians with evidence-based guidance to improve patient outcomes. By adopting double therapy with clopidogrel alone in this specific patient population, healthcare professionals can effectively manage the delicate balance between bleeding and thrombotic risks associated with PCI and oral anticoagulant use.
In conclusion, the WOEST trial has contributed valuable insights into the management of patients undergoing PCI while taking oral anticoagulants. The study’s results highlight the superiority of clopidogrel monotherapy over triple therapy, demonstrating a substantial reduction in bleeding complications without an increased risk of thrombotic events. These findings have the potential to transform clinical practice and improve the safety and efficacy of PCI in this patient population.