WOMAN Trial: The Use of Tranexamic Acid in Reducing Death Due to Post-Partum Haemorrhage
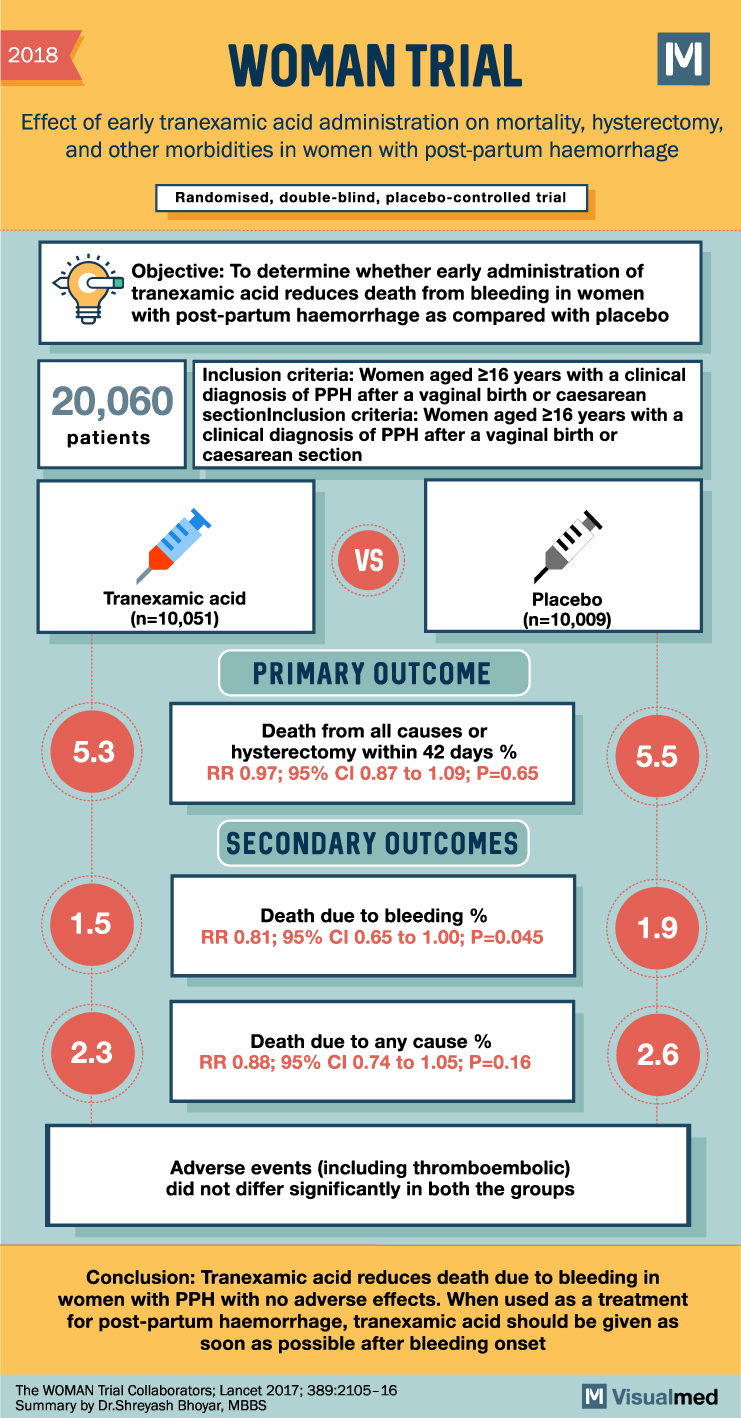
Post-partum haemorrhage (PPH) is a leading cause of maternal mortality worldwide. The administration of tranexamic acid has been shown to decrease bleeding-related deaths in trauma patients. A recent trial aimed to assess the impact of early tranexamic acid administration on death, hysterectomy, and other relevant outcomes in women experiencing PPH.
This randomized, double-blind, placebo-controlled trial (WOMAN trial) included women aged 16 years and older who had been clinically diagnosed with PPH after vaginal birth or cesarean section. Women were randomly assigned to receive either 1g intravenous tranexamic acid or a matching placebo, along with standard care. A second dose could be administered if bleeding persisted after 30 minutes or resumed within 24 hours of the first dose.
The study, which initially aimed to enroll 15,000 women, increased its sample size to 20,060 women to more accurately estimate the effect of tranexamic acid on the risk of death from PPH. The study participants, caregivers, and outcome assessors were all masked to the allocation.
The results showed a significant reduction in death due to bleeding in women who received tranexamic acid (1.5%) compared to those who received a placebo (1.9%). This reduction was particularly evident in women who received treatment within 3 hours of giving birth. However, tranexamic acid did not significantly reduce the rate of hysterectomy nor the composite primary endpoint of death from all causes or hysterectomy. Moreover, adverse events, including thromboembolic events, did not significantly differ between the tranexamic acid and placebo groups.
In conclusion, this study revealed that tranexamic acid can reduce death due to bleeding in women experiencing PPH, without any adverse effects. The findings suggest that tranexamic acid should be administered as early as possible after the onset of bleeding during the post-partum period.