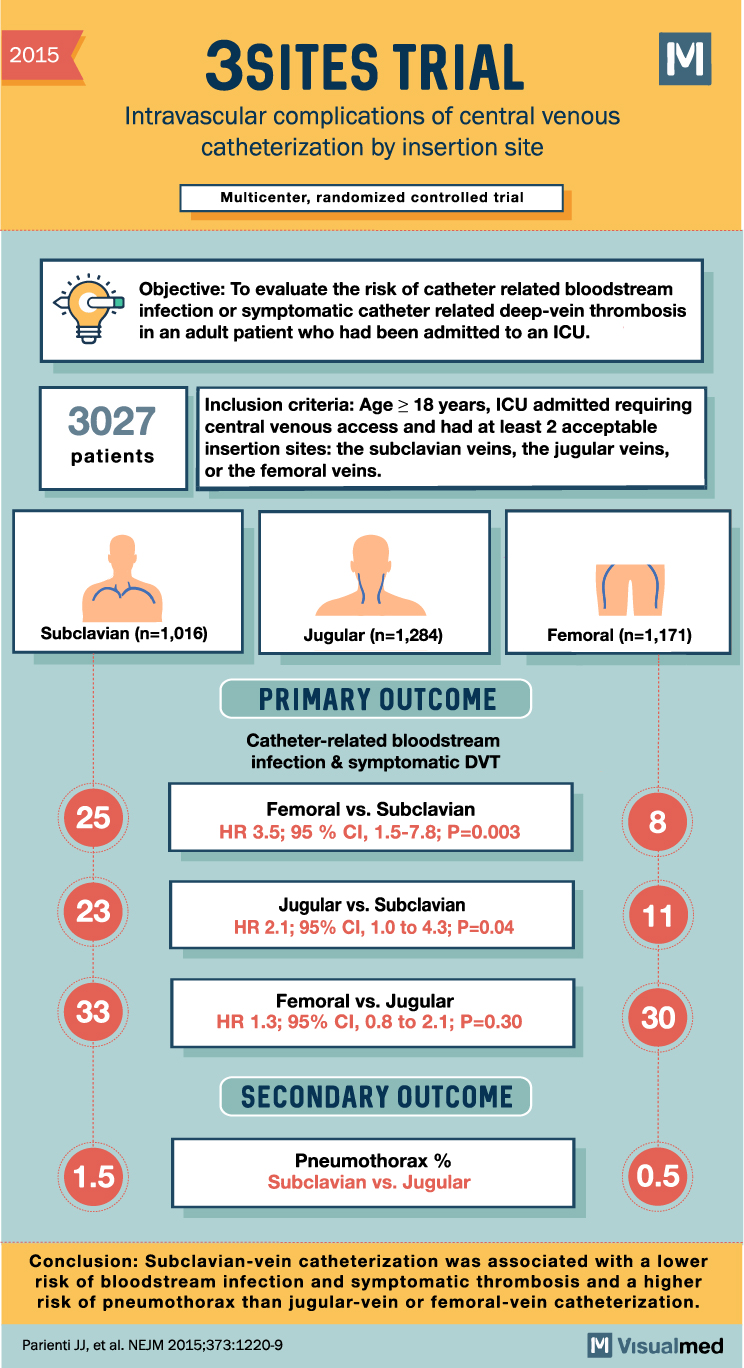
2015 3SITES TRIAL Intravascular complications of central venous catheterization by insertion site Multicenter, randomized controlled trial Objective: To evaluate the risk of catheter related bloodstream infection or symptomatic catheter related deep-vein thrombosis in an adult patient who had been admitted to an ICU. 3027 Inclusion criteria: Age > 18 years, ICU admitted requiring central venous access and had at least 2 acceptable insertion sites: the subclavian veins, the jugular veins, or the femoral veins. patients Subclavian (n=1,016) Jugular (n=1,284) Femoral (n=1,171) PRIMARY OUTCOME Catheter-related bloodstream infection & symptomatic DVT 25 Femoral vs. Subclavian HR 3.5; 95 % CI, 1.5-7.8; P=0.003 Jugular vs. Subclavian HR 2.1; 95% CI, 1.0 to 4.3; P=0.04 33 Femoral vs. Jugular HR 1.3; 95% CI, 0.8 to 2.1; P=0.30 30 SECONDARY OUTCOME Pneumothorax % Subclavian vs. Jugular Conclusion: Subclavian-vein catheterization was associated with a lower risk of bloodstream infection and symptomatic thrombosis and a higher risk of pneumothorax than jugular-vein or femoral-vein catheterization. Parienti JJ, et al. NEJM 2015;373:1220-9